Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice
- PMID: 15876356
- PMCID: PMC1142324
- DOI: 10.1186/1471-2202-6-33
Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice
Abstract
Background: ADAM22 is a member of the ADAM gene family, but the fact that it is expressed only in the nervous systems makes it unique. ADAM22's sequence similarity to other ADAMs suggests it to be an integrin binder and thus to have a role in cell-cell or cell-matrix interactions. To elucidate the physiological functions of ADAM22, we employed gene targeting to generate ADAM22 knockout mice.
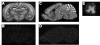
Results: ADAM22-deficient mice were produced in a good accordance with the Mendelian ratio and appeared normal at birth. After one week, severe ataxia was observed, and all homozygotes died before weaning, probably due to convulsions. No major histological abnormalities were detected in the cerebral cortex or cerebellum of the homozygous mutants; however, marked hypomyelination of the peripheral nerves was observed.
Conclusion: The results of our study demonstrate that ADAM22 is closely involved in the correct functioning of the nervous system. Further analysis of ADAM22 will provide clues to understanding the mechanisms of human diseases such as epileptic seizures and peripheral neuropathy.
Figures







Similar articles
-
Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling.J Neurosci. 2010 Mar 10;30(10):3857-64. doi: 10.1523/JNEUROSCI.6287-09.2010. J Neurosci. 2010. PMID: 20220021 Free PMC article.
-
Biological characterization of ADAM22 variants reveals the importance of a disintegrin domain sequence in cell surface expression.J Recept Signal Transduct Res. 2010 Apr;30(2):72-7. doi: 10.3109/10799891003614790. J Recept Signal Transduct Res. 2010. PMID: 20156119
-
Pure axonal neuropathy: nerve xenografts and clinicopathological study of a family with peripheral neuropathy, hereditary ataxia, focal necrotizing encephalopathy, and spongy degeneration of brain.Ann Neurol. 1980 Mar;7(3):251-61. doi: 10.1002/ana.410070308. Ann Neurol. 1980. PMID: 6252824
-
[Epilepsy-related LGI1 functions as a synaptic modulator through ADAM22].Tanpakushitsu Kakusan Koso. 2007 May;52(5):449-55. Tanpakushitsu Kakusan Koso. 2007. PMID: 17491326 Review. Japanese. No abstract available.
-
The LGI1-ADAM22 protein complex in synaptic transmission and synaptic disorders.Neurosci Res. 2017 Mar;116:39-45. doi: 10.1016/j.neures.2016.09.011. Epub 2016 Oct 4. Neurosci Res. 2017. PMID: 27717669 Review.
Cited by
-
ADAM22 ethnic-specific variant reducing binding of membrane-associated guanylate kinases causes focal epilepsy and behavioural disorder.Brain Commun. 2023 Oct 27;5(6):fcad295. doi: 10.1093/braincomms/fcad295. eCollection 2023. Brain Commun. 2023. PMID: 37953841 Free PMC article.
-
How Schwann Cells Sort Axons: New Concepts.Neuroscientist. 2016 Jun;22(3):252-65. doi: 10.1177/1073858415572361. Epub 2015 Feb 16. Neuroscientist. 2016. PMID: 25686621 Free PMC article. Review.
-
ADAM15 gene structure and differential alternative exon use in human tissues.BMC Mol Biol. 2007 Oct 15;8:90. doi: 10.1186/1471-2199-8-90. BMC Mol Biol. 2007. PMID: 17937806 Free PMC article.
-
ADAM2 promotes migration of neuroblasts in the rostral migratory stream to the olfactory bulb.Eur J Neurosci. 2008 Apr;27(7):1585-95. doi: 10.1111/j.1460-9568.2008.06119.x. Eur J Neurosci. 2008. PMID: 18380661 Free PMC article.
-
Clustered K+ channel complexes in axons.Neurosci Lett. 2010 Dec 10;486(2):101-6. doi: 10.1016/j.neulet.2010.08.081. Epub 2010 Sep 17. Neurosci Lett. 2010. PMID: 20816921 Free PMC article. Review.
References
-
- Becherer JD, Blobel CP. Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs) Curr Top Dev Biol. 2003;54:101–123. - PubMed
-
- Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. - PubMed
-
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. - PubMed
-
- Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. - PubMed
-
- Ino M, Yoshinaga T, Wakamori M, Miyamoto N, Takahashi E, Sonoda J, Kagaya T, Oki T, Nagasu T, Nishizawa Y, Tanaka I, Imoto K, Aizawa S, Koch S, Schwartz A, Niidome T, Sawada K, Mori Y. Functional disorders of the sympathetic nervous system in mice lacking the alpha 1B subunit (Cav 2.2) of N-type calcium channels. Proc Natl Acad Sci U S A. 2001;98:5323–5328. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

