Active site of Zn(2+)-dependent sn-glycerol-1-phosphate dehydrogenase from Aeropyrum pernix K1
- PMID: 15876564
- PMCID: PMC2685552
- DOI: 10.1155/2005/257264
Active site of Zn(2+)-dependent sn-glycerol-1-phosphate dehydrogenase from Aeropyrum pernix K1
Abstract
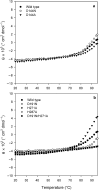
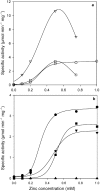
The enzyme sn-glycerol-1-phosphate dehydrogenase (Gro1PDH, EC 1.1.1.261) is key to the formation of the enantiomeric configuration of the glycerophosphate backbone (sn-glycerol-1-phosphate) of archaeal ether lipids. This enzyme catalyzes the reversible conversion between dihydroxyacetone phosphate and glycerol-1-phosphate. To date, no information about the active site and catalytic mechanism of this enzyme has been reported. Using the sequence and structural information for glycerol dehydrogenase, we constructed six mutants (D144N, D144A, D191N, H271A, H287A and D191N/H271A) of Gro1PDH from Aeropyrum pernix K1 and examined their characteristics to clarify the active site of this enzyme. The enzyme was found to be a zinc-dependent metalloenzyme, containing one zinc ion for every monomer protein that was essential for activity. Site-directed mutagenesis of D144 increased the activity of the enzyme. Mutants D144N and D144A exhibited low affinity for the substrates and higher activity than the wild type, but their affinity for the zinc ion was the same as that of the wild type. Mutants D191N, H271A and H287A had a low affinity for the zinc ion and a low activity compared with the wild type. The double mutation, D191N/H271A, had no enzyme activity and bound no zinc. From these results, it was clarified that residues D191, H271 and H287 participate in the catalytic activity of the enzyme by binding the zinc ion, and that D144 has an effect on substrate binding. The structure of the active site of Gro1PDH from A. pernix K1 seems to be similar to that of glycerol dehydrogenase, despite the differences in substrate specificity and biological role.
Figures

Similar articles
-
Catalytic properties and crystal structure of thermostable NAD(P)H-dependent carbonyl reductase from the hyperthermophilic archaeon Aeropyrum pernix K1.Enzyme Microb Technol. 2016 Sep;91:17-25. doi: 10.1016/j.enzmictec.2016.05.008. Epub 2016 May 20. Enzyme Microb Technol. 2016. PMID: 27444325
-
Kinetic study of sn-glycerol-1-phosphate dehydrogenase from the aerobic hyperthermophilic archaeon, Aeropyrum pernix K1.Eur J Biochem. 2002 Feb;269(3):969-76. doi: 10.1046/j.0014-2956.2001.02731.x. Eur J Biochem. 2002. PMID: 11846799
-
Discrimination of esterase and peptidase activities of acylaminoacyl peptidase from hyperthermophilic Aeropyrum pernix K1 by a single mutation.J Biol Chem. 2006 Jul 7;281(27):18618-25. doi: 10.1074/jbc.M601015200. Epub 2006 May 2. J Biol Chem. 2006. PMID: 16670095
-
Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations.Microbiol Mol Biol Rev. 2007 Mar;71(1):97-120. doi: 10.1128/MMBR.00033-06. Microbiol Mol Biol Rev. 2007. PMID: 17347520 Free PMC article. Review.
-
Advances in Physicochemical and Biochemical Characterization of Archaeosomes from Polar Lipids of Aeropyrum pernix K1 and Stability in Biological Systems.ACS Omega. 2023 Jan 13;8(3):2861-2870. doi: 10.1021/acsomega.2c07406. eCollection 2023 Jan 24. ACS Omega. 2023. PMID: 36713696 Free PMC article. Review.
Cited by
-
Enzyme-driven speciation: crystallizing Archaea via lipid capture.J Mol Evol. 2007 Mar;64(3):364-74. doi: 10.1007/s00239-006-0141-8. Epub 2007 Jan 25. J Mol Evol. 2007. PMID: 17253090
-
Mutational analysis of the high-affinity zinc binding site validates a refined human dopamine transporter homology model.PLoS Comput Biol. 2013;9(2):e1002909. doi: 10.1371/journal.pcbi.1002909. Epub 2013 Feb 21. PLoS Comput Biol. 2013. PMID: 23436987 Free PMC article.
-
Biosynthesis of archaeal membrane ether lipids.Front Microbiol. 2014 Nov 26;5:641. doi: 10.3389/fmicb.2014.00641. eCollection 2014. Front Microbiol. 2014. PMID: 25505460 Free PMC article. Review.
-
Structure and Evolution of the Archaeal Lipid Synthesis Enzyme sn-Glycerol-1-phosphate Dehydrogenase.J Biol Chem. 2015 Aug 28;290(35):21690-704. doi: 10.1074/jbc.M115.647461. Epub 2015 Jul 14. J Biol Chem. 2015. PMID: 26175150 Free PMC article.
-
Comparative genomic analysis of evolutionarily conserved but functionally uncharacterized membrane proteins in archaea: Prediction of novel components of secretion, membrane remodeling and glycosylation systems.Biochimie. 2015 Nov;118:302-12. doi: 10.1016/j.biochi.2015.01.004. Epub 2015 Jan 9. Biochimie. 2015. PMID: 25583072 Free PMC article.
References
-
- Daiyasu H., Hiroike T., Koga Y., Toh H. Analysis of membrane stereochemistry with homology modeling of sn-glycerol-1- phosphate dehydrogenase. Protein Eng. 2002;15:987–995. - PubMed
-
- Faguy D.M., Doolittle W.F. Genomics: lessons from the Aeropyrum pernix genome. Curr. Biol. 1999;9:R883–R886. - PubMed
-
- Gomez-Ortiz M., Gomis-Ruth F.X., Huber R., Aviles F.X. Inhibition of carboxypeptidase A by excess zinc: analysis of the structural determinant by X-ray crystallography. FEBS Lett. 1997;400:336–340. - PubMed
-
- Han J.-S., Kosugi Y., Ishida H., Ishikawa K. Kinetic study of sn-glycerol-1-phosphate dehydrogenase from the aerobic hyperthermophilic archaeon, Aeropyrum pernix K1. Eur. J. Biochem. 2002;269:969–976. - PubMed
-
- Kawarabayasi Y., Hiro Y., Horikawa H., et al. Complete genome sequence of an aerobic hyperthermophilic crenarchaeon, Aeropyrumpernix K1. DNA Res. 1999;6:83–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

