Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression
- PMID: 15877228
- PMCID: PMC11032820
- DOI: 10.1007/s00262-005-0703-4
Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression
Abstract
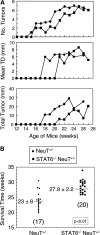
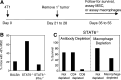
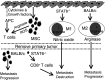
The mouse 4T1 mammary carcinoma is a BALB/c-derived tumor that spontaneously metastasizes and induces immune suppression. Although >95% of wild type BALB/c mice die from metastatic 4T1 tumor even if the primary mammary tumor is surgically removed, >65% of BALB/c mice with a deleted Signal Transducer Activator of Transcription 6 (STAT6) gene survive post-surgery. STAT6-deficiency also confers enhanced immunity against spontaneously developing breast cancer since NeuT+/- mice that are STAT6-deficient develop mammary tumors later and survive longer than NeuT+/- mice that are STAT6-competent. Rejection of metastastic disease and survival of STAT6-deficient mice after removal of primary tumor involve three mechanisms: (1) The generation of M1 type macrophages that produce nitric oxide and are tumoricidal; (2) A decrease to normal in the elevated levels of myeloid suppressor cells that accumulate during primary tumor growth; and (3) CD8+ tumor-specific T lymphocytes. STAT6-deficient, but not wild type BALB/c, mice generate nitric oxide producing macrophages because they lack the STAT6 transcription factor which is necessary for signaling through the type 2 IL-4Ralpha complex, and which induces the production of arginase instead of nitric oxide.
Figures
Similar articles
-
Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease.J Immunol. 2005 Jan 15;174(2):636-45. doi: 10.4049/jimmunol.174.2.636. J Immunol. 2005. PMID: 15634881
-
Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis.Cancer Res. 2005 Dec 15;65(24):11743-51. doi: 10.1158/0008-5472.CAN-05-0045. Cancer Res. 2005. PMID: 16357187
-
Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent.J Immunol. 2002 Nov 15;169(10):5796-804. doi: 10.4049/jimmunol.169.10.5796. J Immunol. 2002. PMID: 12421960
-
Signal transducer and activator of transcription 6 (Stat6) and CD1: inhibitors of immunosurveillance against primary tumors and metastatic disease.Cancer Immunol Immunother. 2004 Feb;53(2):86-91. doi: 10.1007/s00262-003-0446-z. Epub 2003 Oct 30. Cancer Immunol Immunother. 2004. PMID: 14593499 Free PMC article. Review.
-
Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells.Immunol Rev. 2008 Apr;222:162-79. doi: 10.1111/j.1600-065X.2008.00602.x. Immunol Rev. 2008. PMID: 18364001 Review.
Cited by
-
The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis.Immunology. 2011 Jun;133(2):221-38. doi: 10.1111/j.1365-2567.2011.03429.x. Epub 2011 Apr 1. Immunology. 2011. PMID: 21453419 Free PMC article.
-
Mechanical disruption of tumors by iron particles and magnetic field application results in increased anti-tumor immune responses.PLoS One. 2012;7(10):e48049. doi: 10.1371/journal.pone.0048049. Epub 2012 Oct 25. PLoS One. 2012. PMID: 23133545 Free PMC article.
-
Breast cancer cell targeting by prenylation inhibitors elucidated in living animals with a bioluminescence reporter.Clin Cancer Res. 2012 Aug 1;18(15):4136-44. doi: 10.1158/1078-0432.CCR-12-0642. Epub 2012 Jun 12. Clin Cancer Res. 2012. PMID: 22693355 Free PMC article.
-
An obligatory anaerobic Salmonella typhimurium strain redirects M2 macrophages to the M1 phenotype.Oncol Lett. 2018 Mar;15(3):3918-3922. doi: 10.3892/ol.2018.7742. Epub 2018 Jan 8. Oncol Lett. 2018. PMID: 29456740 Free PMC article.
-
Immune heterogeneity in neuroinflammation: dendritic cells in the brain.J Neuroimmune Pharmacol. 2013 Mar;8(1):145-62. doi: 10.1007/s11481-012-9414-8. Epub 2012 Nov 1. J Neuroimmune Pharmacol. 2013. PMID: 23114889 Free PMC article. Review.
References
-
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678. - PubMed
-
- Bantounas I, Phylactou LA, Uney JB. RNA interference and the use of small interfering RNA to study gene function in mammalian systems. J Mol Endocrinol. 2004;33:545. - PubMed
-
- Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De Giovanni C, Bouchard P, Wolf S, Modesti A, Musiani P, Lollini PL, Colombo MP, Forni G. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589. doi: 10.1084/jem.188.3.589. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

