BMP-6 inhibits growth of mature human B cells; induction of Smad phosphorylation and upregulation of Id1
- PMID: 15877825
- PMCID: PMC1134658
- DOI: 10.1186/1471-2172-6-9
BMP-6 inhibits growth of mature human B cells; induction of Smad phosphorylation and upregulation of Id1
Abstract
Background: Bone morphogenetic proteins (BMPs) belong to the TGF-beta superfamily and are secreted proteins with pleiotropic roles in many different cell types. A potential role of BMP-6 in the immune system has been implied by various studies of malignant and rheumatoid diseases. In the present study, we explored the role of BMP-6 in normal human peripheral blood B cells.
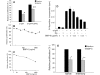
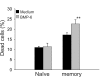
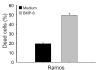
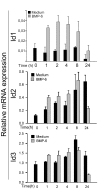
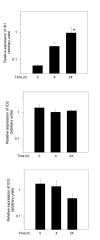
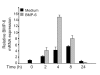
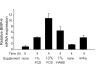
Results: The B cells were found to express BMP type I and type II receptors and BMP-6 rapidly induced phosphorylation of Smad1/5/8. Furthermore, Smad-phosphorylation was followed by upregulation of Id1 mRNA and Id1 protein, whereas Id2 and Id3 expression was not affected. Furthermore, we found that BMP-6 had an antiproliferative effect both in naive (CD19+CD27-) and memory B cells (CD19+CD27+) stimulated with anti-IgM alone or the combined action of anti-IgM and CD40L. Additionally, BMP-6 induced cell death in activated memory B cells. Importantly, the antiproliferative effect of BMP-6 in B-cells was completely neutralized by the natural antagonist, noggin. Furthermore, B cells were demonstrated to upregulate BMP-6 mRNA upon stimulation with anti-IgM.
Conclusion: In mature human B cells, BMP-6 inhibited cell growth, and rapidly induced phosphorylation of Smad1/5/8 followed by an upregulation of Id1.
Figures




Similar articles
-
BMP-6 inhibits human bone marrow B lymphopoiesis--upregulation of Id1 and Id3.Exp Hematol. 2006 Jan;34(1):72-81. doi: 10.1016/j.exphem.2005.09.010. Exp Hematol. 2006. PMID: 16413393
-
Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension.Circ Res. 2008 May 23;102(10):1212-21. doi: 10.1161/CIRCRESAHA.108.173567. Epub 2008 Apr 24. Circ Res. 2008. PMID: 18436795
-
Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo.Circ Res. 2010 Jul 23;107(2):252-62. doi: 10.1161/CIRCRESAHA.109.209940. Epub 2010 Jun 3. Circ Res. 2010. PMID: 20522807
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Controlling cell fate by bone morphogenetic protein receptors.Mol Cell Endocrinol. 2003 Dec 15;211(1-2):105-13. doi: 10.1016/j.mce.2003.09.016. Mol Cell Endocrinol. 2003. PMID: 14656483 Review.
Cited by
-
Aging: a portrait from gene expression profile in blood cells.Aging (Albany NY). 2016 Aug;8(8):1802-21. doi: 10.18632/aging.101016. Aging (Albany NY). 2016. PMID: 27545843 Free PMC article.
-
Effect of bone morphogenetic protein-6 on macrophages.Immunology. 2009 Sep;128(1 Suppl):e442-50. doi: 10.1111/j.1365-2567.2008.02998.x. Epub 2008 Dec 23. Immunology. 2009. PMID: 19191909 Free PMC article.
-
B-cells in pulmonary arterial hypertension: friend, foe or bystander?Eur Respir J. 2024 Apr 25;63(4):2301949. doi: 10.1183/13993003.01949-2023. Print 2024 Apr. Eur Respir J. 2024. PMID: 38485150 Free PMC article. Review.
-
A Tale from TGF-β Superfamily for Thymus Ontogeny and Function.Front Immunol. 2015 Sep 10;6:442. doi: 10.3389/fimmu.2015.00442. eCollection 2015. Front Immunol. 2015. PMID: 26441956 Free PMC article. Review.
-
Therapeutic potential of regulatory cytokines that target B cells.Int Immunol. 2016 Apr;28(4):189-95. doi: 10.1093/intimm/dxv069. Epub 2015 Dec 8. Int Immunol. 2016. PMID: 26647406 Free PMC article. Review.
References
-
- Chen D, Zhao M, Harris SE, Mi Z. Signal transduction and biological functions of bone morphogenetic proteins. Front Biosci. 2004;9:349–358. - PubMed
-
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. - PubMed
-
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

