Cloning, expression, and characterization of the unique bovine A1 adenosine receptor. Studies on the ligand binding site by site-directed mutagenesis
- PMID: 1587851
- PMCID: PMC3463104
Cloning, expression, and characterization of the unique bovine A1 adenosine receptor. Studies on the ligand binding site by site-directed mutagenesis
Abstract
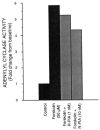
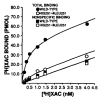
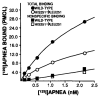
The bovine brain A1 adenosine receptor (A1AR) is distinct from other A1ARs in that it displays the unique agonist potency series of N6-R-phenylisopropyladenosine (R-PIA) greater than N6-S-phenylisopropyladenosine (S-PIA) greater than 5'-N-ethylcarboxamidoadenosine and has a 5-10-fold higher affinity for both agonists and antagonists. The cDNA for this receptor has been cloned from a size-selected (2-4-kb) bovine brain library and sequenced. The 2.0-kb cDNA encodes a protein of 326 amino acid residues with a molecular mass of 36,570 daltons. The amino acid sequence fits well into the seven-transmembrane domain motif typical of G protein-coupled receptors. Northern analysis in bovine tissue using the full length cDNA demonstrates mRNAs of 3.4 and 5.7 kb with a tissue distribution consistent with A1AR binding. Subcloning of the cDNA in a pCMV5 expression vector with subsequent transfection into both COS7 and Chinese hamster ovary cells revealed a fully functional A1AR which could inhibit adenylylcyclase and retained the unique pharmacologic properties of the bovine brain A1AR. The A1AR was found to have a single histidine residue in each of transmembrane domains 6 and 7. Histidine residues have been postulated by biochemical studies to be important for ligand binding. Mutation of His-278 to Leu-278 (seventh transmembrane domain) dramatically decreased both agonist and antagonist binding by greater than 90%. In contrast, mutation of His-251 to Leu-251 decreased antagonist affinity and the number of receptors recognized by an antagonist radioligand. In contrast, agonist affinity was not perturbed but the number of receptors detected by an agonist radioligand was also reduced. These data suggest that both histidines are important for both agonist and antagonist binding, but His-278 appears critical for ligand binding to occur.
Figures





Similar articles
-
Cloning and expression of an A1 adenosine receptor from rat brain.Mol Pharmacol. 1991 Jul;40(1):1-7. Mol Pharmacol. 1991. PMID: 1857334
-
Purification and characterization of bovine cerebral cortex A1 adenosine receptor.Arch Biochem Biophys. 1990 Dec;283(2):440-6. doi: 10.1016/0003-9861(90)90665-l. Arch Biochem Biophys. 1990. PMID: 2275555 Free PMC article.
-
Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor.Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7432-6. doi: 10.1073/pnas.89.16.7432. Proc Natl Acad Sci U S A. 1992. PMID: 1323836 Free PMC article.
-
Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution.Mol Pharmacol. 1993 Sep;44(3):524-32. Mol Pharmacol. 1993. PMID: 8396714
-
Adenosine receptors: protein and gene structure.Arch Int Pharmacodyn Ther. 1995 Jan-Feb;329(1):135-50. Arch Int Pharmacodyn Ther. 1995. PMID: 7639615 Review.
Cited by
-
Functional characterization of the adenosine receptor mediating inhibition of intestinal secretion.Br J Pharmacol. 1995 Jan;114(1):152-6. doi: 10.1111/j.1476-5381.1995.tb14919.x. Br J Pharmacol. 1995. PMID: 7712011 Free PMC article.
-
Immunological identification of A2 adenosine receptors by two antipeptide antibody preparations.Mol Pharmacol. 1992 Sep;42(3):391-7. Mol Pharmacol. 1992. PMID: 1328841 Free PMC article.
-
Molecular Characterization of A(1) and A(2a) Adenosine Receptors.Drug Dev Res. 1993 Mar;28(3):226-231. doi: 10.1002/ddr.430280307. Epub 2004 Oct 5. Drug Dev Res. 1993. PMID: 23002320 Free PMC article.
-
AMP is an adenosine A1 receptor agonist.J Biol Chem. 2012 Feb 17;287(8):5301-9. doi: 10.1074/jbc.M111.291666. Epub 2012 Jan 3. J Biol Chem. 2012. PMID: 22215671 Free PMC article.
-
Molecular Architecture of G Protein-Coupled Receptors.Drug Dev Res. 1996 Jan 1;37(1):1-38. doi: 10.1002/(SICI)1098-2299(199601)37:1<1::AID-DDR1>3.0.CO;2-S. Drug Dev Res. 1996. PMID: 21921973 Free PMC article.
References
-
- Olsson RA, Pearson JD. Physiol. Rev. 1990;70:761–845. - PubMed
-
- Stiles GL. Clin. Res. 1990;38:10–18. - PubMed
-
- Stiles GL. News Physiol. Sci. 1991;6:161–165.
-
- Parsons WJ, Stiles GL. J. Biol. Chem. 1987;262:841–847. - PubMed
-
- Longabaugh JP, Didsbury J, Spiegel A, Stiles GL. Mol. Phurmacol. 1989;36:681–688. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

