Antibody responses to individual proteins of SARS coronavirus and their neutralization activities
- PMID: 15878679
- PMCID: PMC7110836
- DOI: 10.1016/j.micinf.2005.02.006
Antibody responses to individual proteins of SARS coronavirus and their neutralization activities
Abstract
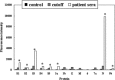
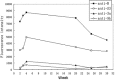
A novel coronavirus, the severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV), was identified as the causative agent of SARS. The profile of specific antibodies to individual proteins of the virus is critical to the development of vaccine and diagnostic tools. In this study, 13 recombinant proteins associated with four structural proteins (S, E, M and N) and five putative uncharacterized proteins (3a, 3b, 6, 7a and 9b) of the SARS-CoV were prepared and used for screening and monitoring their specific IgG antibodies in SARS patient sera by protein microarray. Antibodies to proteins S, 3a, N and 9b were detected in the sera from convalescent-phase SARS patients, whereas those to proteins E, M, 3b, 6 and 7a were undetected. In the detectable specific antibodies, anti-S and anti-N were dominant and could persist in the sera of SARS patients until week 30. Among the rabbit antisera to recombinant proteins S3, N, 3a and 9b, only anti-S3 serum showed significant neutralizing activity to the SARS-CoV infection in Vero E6 cells. The results suggest (1) that anti-S and anti-N antibodies are diagnostic markers and in particular that S3 is immunogenic and therefore is a good candidate as a subunit vaccine antigen; and (2) that, from a virus structure viewpoint, the presence in some human sera of antibodies reacting with two recombinant polypeptides, 3a and 9b, supports the hypothesis that they are synthesized during the virus cycle.
Figures
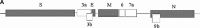
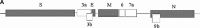

References
-
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. - PubMed
-
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. New Engl. J. Med. 2003;348:1953–1966. - PubMed
-
- Drosten C., Gunther S., Preiser W., Van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New Engl. J. Med. 2003;348:1967–1976. - PubMed
-
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. - PubMed
-
- Qin E., Zhu Q., Yu M., Fan B., Chang G., Si B., Yang B., Fan B., Chang G., Si B., Yang B., Peng W., Jiang T., Liu B., Deng Y., Liu H., Zhang Y., Wang C., Li Y., Gan Y., Li X., Lu F., Tan G., Cao W., Yang R. A complete sequence and comparative analysis of a SARS-associated virus (Isolate BJ01) Chin. Sci. Bull. 2003;48:941–948. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

