In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+CD62L+ T cells which prevent intestinal inflammation in the SCID transfer model of colitis
- PMID: 15879013
- PMCID: PMC1774700
- DOI: 10.1136/gut.2004.046946
In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+CD62L+ T cells which prevent intestinal inflammation in the SCID transfer model of colitis
Abstract
Background and methods: Cytosin-guanosin dinucleotide (CpG) motifs of bacterial DNA are known to be potent activators of innate immunity. We have shown previously that administration of CpG containing oligodeoxynucleotide (CpG-ODN) to mice before the onset of dextran sodium sulphate induced colitis ameliorated colitis and inhibited induction of proinflammatory cytokines. To investigate the possible involvement of CD4(+) T cells in the prophylactic CpG-ODN effects, we used the SCID transfer model of colitis.
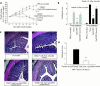
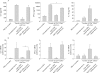
Results: CD4(+)CD62L(+) T cells from CpG-ODN treated donors did not induce significant intestinal inflammation in SCID recipients, in contrast with control cells. Additionally, cotransfer of these cells with CD4(+)CD62L(+) cells from normal mice protected recipient animals from colitis, indicating regulatory activity. Also, CD4(+)CD62L(+) cells from toll-like receptor 9 deficient animals induced a significantly more severe colitis in SCID recipients than cells from wild-type littermate controls, suggesting a similar protective role of "endogenous" bacterial DNA leading to a less "aggressive" phenotype of these cells. There was no detectable difference in regulatory T cell surface markers between aggressive and attenuated cell pools but attenuated cell pools showed reduced proliferation in vitro and in vivo and produced less interferon gamma, interleukin (IL)-5, and IL-6 after anti-CD3 stimulation.
Conclusions: Collectively, our data support the concept that both endogenous bacterial DNA and exogenously supplied CpG motifs of bacterial DNA induce regulatory properties in CD4(+) T cells. Therefore, bacterial DNA derived from the normal gut flora may contribute essentially to the homeostasis between effector and regulatory immune mechanisms in healthy individuals to protect them from chronic intestinal inflammation.
Figures

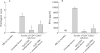

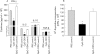
References
-
- Andus T, Gross V. Etiology and pathophysiology of inflammatory bowel disease—environmental factors. Hepatogastroenterology 2000;47:29–43. - PubMed
-
- Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol 1997;92 (12 suppl) :5–11S. - PubMed
-
- Brimnes J, Reimann J, Nissen M, et al. Enteric bacterial antigens activate CD4(+) T cells from scid mice with inflammatory bowel disease. Eur J Immunol 2001;31:23–31. - PubMed
-
- Madsen KL, Malfair D, Gray D, et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis 1999;5:262–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
