Boswellia carterii extract inhibits TH1 cytokines and promotes TH2 cytokines in vitro
- PMID: 15879017
- PMCID: PMC1112084
- DOI: 10.1128/CDLI.12.5.575-580.2005
Boswellia carterii extract inhibits TH1 cytokines and promotes TH2 cytokines in vitro
Abstract
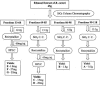
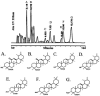
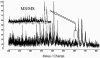
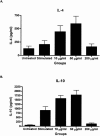
Traditional herbal formulas used to treat inflammatory arthritis in China and India include Boswellia carterii or Boswellia serrata. They both contain boswellic acids (BAs) which have been shown to exhibit anti-inflammatory and antiarthritic properties. This study tests the hypothesis that mixtures of BAs derived from B. carterii have immunomodulatory properties. B. carterii plant resin obtained from China was prepared as an ethanol extract, and the presence of seven BAs was confirmed by column chromatography, high-performance liquid chromatography, and UV laser desorption/ionization tandem mass spectroscopy. The extract was then tested for its ability to alter in vitro production of TH1 cytokines (interleukin-2 [IL-2] and gamma interferon) and TH2 cytokines (IL-4 and IL-10) by murine splenocytes. Delivery of the resin extract using ethanol as a solvent resulted in significant cellular toxicity not seen with the addition of ethanol alone. By contrast, delivery of the resin extract using a sesame oil solvent resulted in a dose-dependent inhibition of TH1 cytokines coupled with a dose-dependent potentiation of TH2 cytokines. These results indicate that a purified mixture of BAs from B. carterii plant resin exhibits carrier-dependent immunomodulatory properties in vitro.
Figures
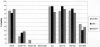

References
-
- Ammon, H. P. 2002. Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory diseases. Wien. Med. Wochenschr. 152:373-378. (In German.) - PubMed
-
- Ammon, H. P. 1996. Salai guggal—Boswellia serrata: from a herbal medicine to a non-redox inhibitor of leukotriene biosynthesis. Eur. J. Med. Res. 1:369-370. - PubMed
-
- Cohen, L. H., and A. I. Gusev. 2002. Small molecule analysis by MALDI mass spectrometry. Anal. Bioanal. Chem. 373:571-586. - PubMed
-
- Coppens, J. J. W. 1995. Olibanum (frankincense), myrrh and opopapnax resins and oils, p. 111. In Flavors and fragrances of plant origin. Non-wood forest products, vol. 1. Food and Agriculture Organization of the United Nations, Rome, Italy.
-
- Culioli, G., C. Mathe, P. Archer, and C. Vieillescazes. 2003. A lupane triterpene from frankincense (Boswellia sp., Burseraceae). Phytochemistry 62:537-541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

