Computational thermostabilization of an enzyme
- PMID: 15879217
- PMCID: PMC3412875
- DOI: 10.1126/science.1107387
Computational thermostabilization of an enzyme
Abstract
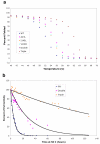
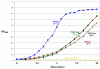
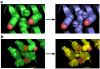
Thermostabilizing an enzyme while maintaining its activity for industrial or biomedical applications can be difficult with traditional selection methods. We describe a rapid computational approach that identified three mutations within a model enzyme that produced a 10 degrees C increase in apparent melting temperature T(m) and a 30-fold increase in half-life at 50 degrees C, with no reduction in catalytic efficiency. The effects of the mutations were synergistic, giving an increase in excess of the sum of their individual effects. The redesigned enzyme induced an increased, temperature-dependent bacterial growth rate under conditions that required its activity, thereby coupling molecular and metabolic engineering.
Figures
References
-
- Haldane JBS. In: Enzymes. Longmans G. a. C., editor. MIT Press; Cambridge: 1930.
-
- Kraut DA, Carroll KS, Herschlag D. Annu Rev Biochem. 2003;72:517. - PubMed
-
- Daniel RM, Dunn RV, Finney JL, Smith JC. Annu Rev Biophys Biomol Struct. 2003;32:69. - PubMed
-
- Eijsink VG, et al. J Biotechnol. 2004 Sep 30;113:105. - PubMed
-
- Scandurra R, Consalvi V, Chiaraluce R, Politi L, Engel PC. Biochimie. 1998 Nov;80:933. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

