Functional properties of mouse connexin30.2 expressed in the conduction system of the heart
- PMID: 15879306
- PMCID: PMC3657762
- DOI: 10.1161/01.RES.0000169271.33675.05
Functional properties of mouse connexin30.2 expressed in the conduction system of the heart
Abstract
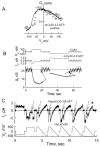
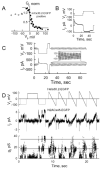
Gap junction channels composed of connexin (Cx) 40, Cx43, and Cx45 proteins are known to be necessary for impulse propagation through the heart. Here, we report mouse connexin30.2 (mCx30.2) to be a new cardiac connexin that is expressed mainly in the conduction system of the heart. Antibodies raised to the cytoplasmic loop or the C-terminal regions of mCx30.2 recognized this protein in mouse heart as well as in HeLa cells transfected with wild-type mCx30.2 or mCx30.2 fused with enhanced green fluorescent protein (mCx30.2-EGFP). Immunofluorescence analyses of adult hearts yielded positive signals within the sinoatrial node, atrioventricular node, and A-V bundle of the cardiac conduction system. Dye transfer studies demonstrated that mCx30.2 and mCx30.2-EGFP channels discriminate poorly on the basis of charge, but do not allow permeation of tracers >400 Da. Both mCx30.2 and mCx30.2-EGFP gap junctional channels exhibited weak sensitivity to transjunctional voltage (Vj) and a single channel conductance of approximately 9 pS, which is the lowest among all members of the connexin family measured in HeLa cell transfectants. HeLa mCx30.2-EGFP transfectants when paired with cells expressing Cx40, Cx43, or Cx45 formed functional heterotypic gap junction channels that exhibited low unitary conductances (15 to 18 pS), rectifying open channel I-V relations and asymmetric Vj dependence. The electrical properties of homo- and hetero-typic junctions involving mCx30.2 may contribute to slow propagation velocity in nodal tissues and directional asymmetry of excitation spread in the AV nodal region.
Figures
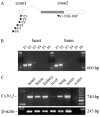
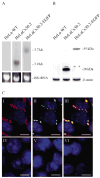
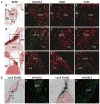

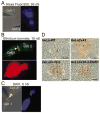

References
-
- Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. - PubMed
-
- Gros DB, Jongsma HJ. Connexins in mammalian heart function. Bioessays. 1996;18:719–730. - PubMed
-
- Severs NJ, Dupont E, Coppen SR, Halliday D, Inett E, Baylis D, Rothery S. Remodelling of gap junctions and connexin expression in heart disease. Biochim Biophys Acta. 2004;1662:138–148. - PubMed
-
- Lo CW. Role of gap junctions in cardiac conduction and development: insights from the connexin knockout mice. Circ Res. 2000;87:346–348. comment] [editorial. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

