The molecular chaperone Sse1 and the growth control protein kinase Sch9 collaborate to regulate protein kinase A activity in Saccharomyces cerevisiae
- PMID: 15879503
- PMCID: PMC1451167
- DOI: 10.1534/genetics.105.043109
The molecular chaperone Sse1 and the growth control protein kinase Sch9 collaborate to regulate protein kinase A activity in Saccharomyces cerevisiae
Abstract
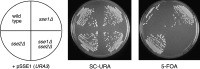
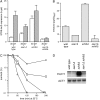
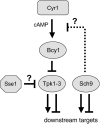
The Sch9 protein kinase regulates Hsp90-dependent signal transduction activity in the budding yeast Saccharomyces cerevisiae. Hsp90 functions in concert with a number of cochaperones, including the Hsp110 homolog Sse1. In this report, we demonstrate a novel synthetic genetic interaction between SSE1 and SCH9. This interaction was observed specifically during growth at elevated temperature and was suppressed by decreased signaling through the protein kinase A (PKA) signal transduction pathway. Correspondingly, sse1Delta sch9Delta cells were shown by both genetic and biochemical approaches to have abnormally high levels of PKA activity and were less sensitive to modulation of PKA by glucose availability. Growth defects of an sse1Delta mutant were corrected by reducing PKA signaling through overexpression of negative regulators or growth on nonoptimal carbon sources. Hyperactivation of the PKA pathway through expression of a constitutive RAS2 allele likewise resulted in temperature-sensitive growth, suggesting that modulation of PKA activity during thermal stress is required for adaptation and viability. Together these results demonstrate that the Sse1 chaperone and the growth control kinase Sch9 independently contribute to regulation of PKA signaling.
Figures






Similar articles
-
PKA and Sch9 control a molecular switch important for the proper adaptation to nutrient availability.Mol Microbiol. 2005 Feb;55(3):862-80. doi: 10.1111/j.1365-2958.2004.04429.x. Mol Microbiol. 2005. PMID: 15661010
-
In Saccharomyces cerevisiae an unbalanced level of tyrosine phosphorylation down-regulates the Ras/PKA pathway.Int J Biochem Cell Biol. 2006 Mar;38(3):444-60. doi: 10.1016/j.biocel.2005.10.004. Epub 2005 Nov 2. Int J Biochem Cell Biol. 2006. PMID: 16297653
-
The Hsp110 protein chaperone Sse1 is required for yeast cell wall integrity and morphogenesis.Curr Genet. 2008 Jul;54(1):1-11. doi: 10.1007/s00294-008-0193-y. Epub 2008 May 14. Curr Genet. 2008. PMID: 18478233 Free PMC article.
-
Glucose-stimulated cAMP-protein kinase A pathway in yeast Saccharomyces cerevisiae.J Biosci Bioeng. 2007 Oct;104(4):245-50. doi: 10.1263/jbb.104.245. J Biosci Bioeng. 2007. PMID: 18023794 Review.
-
Saccharomyces cerevisiae plasma membrane nutrient sensors and their role in PKA signaling.FEMS Yeast Res. 2010 Mar;10(2):134-49. doi: 10.1111/j.1567-1364.2009.00587.x. Epub 2009 Oct 17. FEMS Yeast Res. 2010. PMID: 19849717 Review.
Cited by
-
Mutational analysis of Sse1 (Hsp110) suggests an integral role for this chaperone in yeast prion propagation in vivo.G3 (Bethesda). 2013 Aug 7;3(8):1409-18. doi: 10.1534/g3.113.007112. G3 (Bethesda). 2013. PMID: 23797105 Free PMC article.
-
Flo11p-independent control of "mat" formation by hsp70 molecular chaperones and nucleotide exchange factors in yeast.Genetics. 2007 Nov;177(3):1679-89. doi: 10.1534/genetics.107.081141. Epub 2007 Oct 18. Genetics. 2007. PMID: 17947402 Free PMC article.
-
Prion aggregate structure in yeast cells is determined by the Hsp104-Hsp110 disaggregase machinery.J Cell Biol. 2015 Oct 12;211(1):145-58. doi: 10.1083/jcb.201505104. Epub 2015 Oct 5. J Cell Biol. 2015. PMID: 26438827 Free PMC article.
-
Substrate binding by the yeast Hsp110 nucleotide exchange factor and molecular chaperone Sse1 is not obligate for its biological activities.Mol Biol Cell. 2017 Jul 15;28(15):2066-2075. doi: 10.1091/mbc.E17-01-0070. Epub 2017 May 24. Mol Biol Cell. 2017. PMID: 28539411 Free PMC article.
-
Interactions between the kinetochore complex and the protein kinase A pathway in Saccharomyces cerevisiae.G3 (Bethesda). 2012 Jul;2(7):831-41. doi: 10.1534/g3.112.002675. Epub 2012 Jul 1. G3 (Bethesda). 2012. PMID: 22870406 Free PMC article.
References
-
- Bourbon, H. M., A. Aguilera, A. Z. Ansari, F. J. Asturias, A. J. Berk et al., 2004. A unified nomenclature for protein subunits of mediator complexes linking transcriptional regulators to RNA polymerase II. Mol. Cell 14 553–557. - PubMed
-
- Boy-Marcotte, E., G. Lagniel, M. Perrot, F. Bussereau, A. Boudsocq et al., 1999. The heat shock response in yeast: differential regulations and contributions of the Msn2p/Msn4p and Hsf1p regulons. Mol. Microbiol. 33 274–283. - PubMed
-
- Brodsky, J. L., E. D. Werner, M. E. Dubas, J. L. Goeckeler, K. B. Kruse et al., 1999. The requirement for molecular chaperones during endoplasmic reticulum-associated protein degradation demonstrates that protein export and import are mechanistically distinct. J. Biol. Chem. 274 3453–3460. - PubMed
-
- Broek, D., T. Toda, T. Michaeli, L. Levin, C. Birchmeier et al., 1987. The S. cerevisiae CDC25 gene product regulates the Ras/adenylate cyclase pathway. Cell 48 789–799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

