Class I histone deacetylase Thd1p affects nuclear integrity in Tetrahymena thermophila
- PMID: 15879532
- PMCID: PMC1140101
- DOI: 10.1128/EC.4.5.981-990.2005
Class I histone deacetylase Thd1p affects nuclear integrity in Tetrahymena thermophila
Abstract
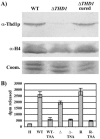
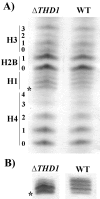
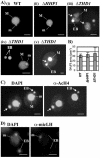
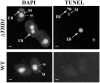
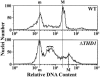
Class I histone deacetylases (HDACs) participate in the regulation of DNA-templated processes such as transcription and replication. Members of this class can act locally at specific sites, or they can act more globally, contributing to a baseline acetylation state, both of which actions may be important for genome maintenance and organization. We previously identified a macronuclear-specific class I HDAC in Tetrahymena thermophila called Thd1p, which is expressed early in the development of the macronucleus when it initially becomes transcriptionally active. To test the idea that Thd1p is important for global chromatin integrity in an active macronucleus, Tetrahymena cells reduced in expression of Thd1p were generated. We observed phenotypes that indicated loss of chromatin integrity in the mutant cells, including DNA fragmentation and extrusion of chromatin from the macronucleus, variable macronuclear size and shape, enlarged nucleoli, and reduced phosphorylation of histone H1 from bulk chromatin. Macronuclei in mutant cells also contained more DNA. This observation suggests a role for Thd1p in the control of nuclear DNA content, a previously undescribed role for class I HDACs. Together, these phenotypes implicate Thd1p in the maintenance of macronuclear integrity in multiple ways, probably through site-specific changes in histone acetylation since no change in the acetylation levels of bulk histones was detected in mutant cells.
Figures



Similar articles
-
Class I histone deacetylase Thd1p promotes global chromatin condensation in Tetrahymena thermophila.Eukaryot Cell. 2007 Oct;6(10):1913-24. doi: 10.1128/EC.00217-07. Epub 2007 Aug 22. Eukaryot Cell. 2007. PMID: 17715364 Free PMC article.
-
Developmentally regulated rpd3p homolog specific to the transcriptionally active macronucleus of vegetative Tetrahymena thermophila.Mol Cell Biol. 2000 Nov;20(22):8319-28. doi: 10.1128/MCB.20.22.8319-8328.2000. Mol Cell Biol. 2000. PMID: 11046129 Free PMC article.
-
Tetrahymena thermophila JMJD3 homolog regulates H3K27 methylation and nuclear differentiation.Eukaryot Cell. 2012 May;11(5):601-14. doi: 10.1128/EC.05290-11. Epub 2012 Mar 16. Eukaryot Cell. 2012. PMID: 22427430 Free PMC article.
-
Nuclear matrix, dynamic histone acetylation and transcriptionally active chromatin.Mol Biol Rep. 1997 Aug;24(3):197-207. doi: 10.1023/a:1006811817247. Mol Biol Rep. 1997. PMID: 9291093 Review.
-
Bromodomain-containing proteins in the unicellular eukaryote Tetrahymena thermophila.Zool Res. 2025 May 18;46(3):538-550. doi: 10.24272/j.issn.2095-8137.2025.011. Zool Res. 2025. PMID: 40259734 Free PMC article. Review.
Cited by
-
Disruption of a ∼23-24 nucleotide small RNA pathway elevates DNA damage responses in Tetrahymena thermophila.Mol Biol Cell. 2021 Jul 15;32(15):1335-1346. doi: 10.1091/mbc.E20-10-0631. Epub 2021 May 19. Mol Biol Cell. 2021. PMID: 34010017 Free PMC article.
-
The condensin complex is essential for amitotic segregation of bulk chromosomes, but not nucleoli, in the ciliate Tetrahymena thermophila.Mol Cell Biol. 2006 Jun;26(12):4690-700. doi: 10.1128/MCB.02315-05. Mol Cell Biol. 2006. PMID: 16738332 Free PMC article.
-
Sirtuin-mediated nuclear differentiation and programmed degradation in Tetrahymena.BMC Cell Biol. 2011 Sep 21;12:40. doi: 10.1186/1471-2121-12-40. BMC Cell Biol. 2011. PMID: 21933443 Free PMC article.
-
Transcriptome analysis of the binucleate ciliate Tetrahymena thermophila with asynchronous nuclear cell cycles.Mol Biol Cell. 2023 Feb 1;34(2):rs1. doi: 10.1091/mbc.E22-08-0326. Epub 2022 Dec 7. Mol Biol Cell. 2023. PMID: 36475712 Free PMC article.
-
Class I histone deacetylase Thd1p promotes global chromatin condensation in Tetrahymena thermophila.Eukaryot Cell. 2007 Oct;6(10):1913-24. doi: 10.1128/EC.00217-07. Epub 2007 Aug 22. Eukaryot Cell. 2007. PMID: 17715364 Free PMC article.
References
-
- Allis, C. D., and D. K. Dennison. 1982. Identification and purification of young macronuclear anlagen from conjugating cells of Tetrahymena thermophila. Dev. Biol. 93:519-533. - PubMed
-
- Boulton, S. J., A. Gartner, J. Reboul, P. Vaglio, N. Dyson, D. E. Hill, and M. Vidal. 2002. Combined functional genomic maps of the C. elegans DNA damage response. Science 295:127-131. - PubMed
-
- Brownell, J. E., and C. D. Allis. 1996. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr. Opin. Genet. Dev. 6:176-184. - PubMed
-
- Bruns, P. J., and D. Cassidy-Hanley. 2000. Biolistic transformation of macro- and micronuclei. Methods Cell Biol. 62:501-512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

