Polarity of the mouse embryo is established at blastocyst and is not prepatterned
- PMID: 15879556
- PMCID: PMC1091742
- DOI: 10.1101/gad.1304805
Polarity of the mouse embryo is established at blastocyst and is not prepatterned
Abstract
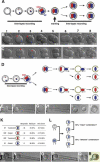
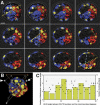
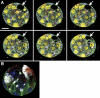
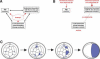
Polarity formation in mammalian preimplantation embryos has long been a subject of controversy. Mammalian embryos are highly regulative, which has led to the conclusion that polarity specification does not exist until the blastocyst stage; however, some recent reports have now suggested polarity predetermination in the egg. Our recent time-lapse recordings have demonstrated that the first cleavage plane is not predetermined in the mouse egg. Here we show that, in contrast to previous claims, two-cell blastomeres do not differ and their precise future contribution to the inner cell mass and/or the trophectoderm cannot be anticipated. Thus, all evidence so far strongly suggests the absence of predetermined axes in the mouse egg. We observe that the ellipsoidal zona pellucida exerts mechanical pressure and space constraints as the coalescing multiple cavities are restricted to one end of the long axis of the blastocyst. We propose that these mechanical cues, in conjunction with the epithelial seal in the outer cell layer, lead to specification of the embryonic-abembryonic axis, thus establishing first polarity in the mouse embryo.
Figures


Comment in
-
Weaknesses in the case against prepatterning in the mouse.Reprod Biomed Online. 2006 Feb;12(2):144-9. doi: 10.1016/s1472-6483(10)60853-x. Reprod Biomed Online. 2006. PMID: 16478575
References
-
- Alarcon V.B. and Marikawa, Y. 2003. Deviation of the blastocyst axis from the first cleavage plane does not affect the quality of mouse postimplantation development. Biol. Reprod. 69: 1208–1212. - PubMed
-
- Aziz M. and Alexandre, H. 1991. The origin of the nascent blastocoele in preimplantation mouse embryos: Ultrastructural cytochemistry and effect of chloroquine. Roux's Arch. Dev. Biol. 200: 77–85. - PubMed
-
- Beddington R.S. and Robertson, E.J. 1999. Axis development and early asymmetry in mammals. Cell 96: 195–209. - PubMed
-
- Calarco P.G. and Brown, E.H. 1969. An ultrastructural and cytological study of preimplantation development of the mouse. J. Exp. Zool. 171: 253–283. - PubMed
-
- Chroscicka A., Komorowski, S., and Maleszewski, M. 2004. Both blastomeres of the mouse 2-cell embryo contribute to the embryonic portion of the blastocyst. Mol. Reprod. Dev. 68: 308–312. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
