Overexpression of fasciculation and elongation protein zeta-1 (FEZ1) induces a post-entry block to retroviruses in cultured cells
- PMID: 15879557
- PMCID: PMC1091744
- DOI: 10.1101/gad.1290005
Overexpression of fasciculation and elongation protein zeta-1 (FEZ1) induces a post-entry block to retroviruses in cultured cells
Abstract
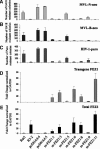
Two mutant Rat2 fibroblast cell lines, R3-2 and R4-7, have been previously isolated by a selection for retrovirus resistance. We have now further analyzed the basis of the block to retroviral infection in the R3-2 line. Using Affymetrix GeneChip analysis, several genes were identified as differentially expressed in the mutant R3-2 line compared with the wild-type cells. One of the candidate gene products, FEZ1 (fasciculation and elongation protein zeta-1), a protein kinase C (PKC)zeta-interacting protein homologous to the Caenorhabditis elegans synaptic transport protein UNC-76, was found to be up-regulated >30-fold in the resistant R3-2 line. FEZ1 overexpression in Rat2 cells conferred a potent resistance to infection by genetically marked retroviruses, and the degree of retroviral resistance in both Rat2 fibroblasts and 293T cells tightly correlated with the expression level of FEZ1 transcripts. FEZ1-overexpressing Rat2 cells showed a similar phenotype to that of the mutant R3-2 line: Infection resulted in normal viral DNA synthesis but a reduction in the formation of circular DNA, indicating a block after reverse transcription but before nuclear entry. Partial knockdown of FEZ1 expression in R3-2 by RNA interference (RNAi) significantly reduced the resistance of this line to infection. Thus, our data suggest that FEZ1 overexpression is sufficient to explain the resistant phenotype of R3-2 cells and identify FEZ1 as a new gene capable of causing retrovirus resistance.
Figures




Similar articles
-
The brain-specific factor FEZ1 is a determinant of neuronal susceptibility to HIV-1 infection.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14040-5. doi: 10.1073/pnas.0900502106. Epub 2009 Aug 10. Proc Natl Acad Sci U S A. 2009. PMID: 19667186 Free PMC article.
-
Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta-interacting protein.J Cell Biol. 1999 Feb 8;144(3):403-11. doi: 10.1083/jcb.144.3.403. J Cell Biol. 1999. PMID: 9971736 Free PMC article.
-
Expression of fasciculation and elongation protein zeta-1 (FEZ1) in cultured rat neonatal astrocytes.Mol Cell Biochem. 2009 May;325(1-2):159-67. doi: 10.1007/s11010-009-0030-8. Epub 2009 Feb 8. Mol Cell Biochem. 2009. PMID: 19199094
-
Functions of fasciculation and elongation protein zeta-1 (FEZ1) in the brain.ScientificWorldJournal. 2010 Aug 17;10:1646-54. doi: 10.1100/tsw.2010.151. ScientificWorldJournal. 2010. PMID: 20730382 Free PMC article. Review.
-
Novel schizophrenia risk factor pathways regulate FEZ1 to advance oligodendroglia development.Transl Psychiatry. 2017 Dec 18;7(12):1293. doi: 10.1038/s41398-017-0028-z. Transl Psychiatry. 2017. PMID: 29249816 Free PMC article. Review.
Cited by
-
FEZ1 phosphorylation regulates HSPA8 localization and interferon-stimulated gene expression.Cell Rep. 2022 Feb 15;38(7):110396. doi: 10.1016/j.celrep.2022.110396. Cell Rep. 2022. PMID: 35172151 Free PMC article.
-
Antiretroviral restriction factors.Curr Opin Virol. 2011 Dec;1(6):526-32. doi: 10.1016/j.coviro.2011.10.007. Curr Opin Virol. 2011. PMID: 22278313 Free PMC article. Review.
-
Moesin regulates stable microtubule formation and limits retroviral infection in cultured cells.EMBO J. 2007 Jan 10;26(1):41-52. doi: 10.1038/sj.emboj.7601475. Epub 2006 Dec 14. EMBO J. 2007. PMID: 17170707 Free PMC article.
-
PDZD8 is a novel Gag-interacting factor that promotes retroviral infection.J Virol. 2010 Sep;84(17):8990-5. doi: 10.1128/JVI.00843-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573829 Free PMC article.
-
Controllable expansion of primary cardiomyocytes by reversible immortalization.Hum Gene Ther. 2009 Dec;20(12):1687-96. doi: 10.1089/hum.2009.057. Hum Gene Ther. 2009. PMID: 19708763 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
