Dual role for tomato heat shock protein 21: protecting photosystem II from oxidative stress and promoting color changes during fruit maturation
- PMID: 15879560
- PMCID: PMC1143080
- DOI: 10.1105/tpc.105.031914
Dual role for tomato heat shock protein 21: protecting photosystem II from oxidative stress and promoting color changes during fruit maturation
Abstract
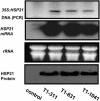
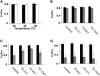
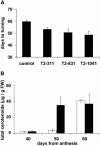
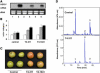
The tomato (Lycopersicon esculentum) chloroplast small heat shock protein (sHSP), HSP21, is induced by heat treatment in leaves, but also under normal growth conditions in developing fruits during the transition of chloroplasts to chromoplasts. We used transgenic tomato plants constitutively expressing HSP21 to study the role of the protein under stress conditions and during fruit maturation. Although we did not find any effect for the transgene on photosystem II (PSII) thermotolerance, our results show that the protein protects PSII from temperature-dependent oxidative stress. In addition, we found direct evidence of the protein's role in fruit reddening and the conversion of chloroplasts to chromoplasts. When plants were grown under normal growth temperature, transgenic fruits accumulated carotenoids earlier than controls. Furthermore, when detached mature green fruits were stored for 2 weeks at 2 degrees C and then transferred to room temperature, the natural accumulation of carotenoids was blocked. In a previous study, we showed that preheat treatment, which induces HSP21, allowed fruit color change at room temperature, after a cold treatment. Here, we show that mature green transgenic fruits constitutively expressing HSP21 do not require the heat treatment to maintain the ability to accumulate carotenoids after cold storage. This study demonstrates that a sHSP plays a role in plant development under normal growth conditions, in addition to its protective effect under stress conditions.
Figures


Similar articles
-
Plastid-expressed AdDjSKI enhances photosystem II stability, delays leaf senescence, and increases fruit yield in tomato plants under heat stress.Physiol Plant. 2024 May-Jun;176(3):e14374. doi: 10.1111/ppl.14374. Physiol Plant. 2024. PMID: 38837422
-
Identification of core subunits of photosystem II as action sites of HSP21, which is activated by the GUN5-mediated retrograde pathway in Arabidopsis.Plant J. 2017 Mar;89(6):1106-1118. doi: 10.1111/tpj.13447. Epub 2017 Feb 27. Plant J. 2017. PMID: 27943531
-
The role of SlCHRC in carotenoid biosynthesis and plastid development in tomato fruit.Int J Biol Macromol. 2024 Nov;281(Pt 3):136354. doi: 10.1016/j.ijbiomac.2024.136354. Epub 2024 Oct 6. Int J Biol Macromol. 2024. PMID: 39378920
-
Regulation of carotenoid formation during tomato fruit ripening and development.J Exp Bot. 2002 Oct;53(377):2107-13. doi: 10.1093/jxb/erf059. J Exp Bot. 2002. PMID: 12324534 Review.
-
Regulation of carotenoid metabolism in tomato.Mol Plant. 2015 Jan;8(1):28-39. doi: 10.1016/j.molp.2014.11.006. Epub 2014 Dec 11. Mol Plant. 2015. PMID: 25578270 Review.
Cited by
-
Integration of QTL, Transcriptome and Polymorphism Studies Reveals Candidate Genes for Water Stress Response in Tomato.Genes (Basel). 2020 Aug 7;11(8):900. doi: 10.3390/genes11080900. Genes (Basel). 2020. PMID: 32784535 Free PMC article.
-
Isolation and characterization of a lipid transfer protein expressed in ripening fruit of Capsicum chinense.Planta. 2006 Mar;223(4):672-83. doi: 10.1007/s00425-005-0120-0. Epub 2005 Sep 22. Planta. 2006. PMID: 16177913
-
Transgenic tomatoes for abiotic stress tolerance: status and way ahead.3 Biotech. 2019 Apr;9(4):143. doi: 10.1007/s13205-019-1665-0. Epub 2019 Mar 18. 3 Biotech. 2019. PMID: 30944790 Free PMC article.
-
Transcriptional Analysis of Carotenoids Accumulation and Metabolism in a Pink-Fleshed Lemon Mutant.Genes (Basel). 2020 Oct 30;11(11):1294. doi: 10.3390/genes11111294. Genes (Basel). 2020. PMID: 33143225 Free PMC article.
-
Physiological and transcriptomic analyses characterized high temperature stress response mechanisms in Sorbus pohuashanensis.Sci Rep. 2021 May 12;11(1):10117. doi: 10.1038/s41598-021-89418-7. Sci Rep. 2021. PMID: 33980903 Free PMC article.
References
-
- Allen, D.J., and Ort, D.R. (2001). Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 6, 36–42. - PubMed
-
- Almoguera, C., Prieto-Dapena, P., and Jordano, J. (1998). Dual regulation of a heat shock promoter during embryogenesis: Stage-dependent role of heat shock elements. Plant J. 13, 437–446. - PubMed
-
- Andersson, B., and Aro, E.-M. (2001). Photodamage and D1 protein turnover in photosystem II. In Advances in Photosynthesis and Respiration, Regulation of Photosynthesis, Vol. 11, E.-M. Aro and B. Andersson, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 377–393.
-
- Asada, K. (1996). Radical production and scavenging in the chloroplasts. In Molecular Biology of Free Radical Scavenging Systems, Vol. 5, N.R. Baker, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 123–150.
-
- Broido, S., Loyter, A., and Vainstein, A. (1991). Expression of plant genes in transfected mammalian cells: Accumulation of recombinant preLHCIIb proteins within cytoplasmic inclusion bodies. Exp. Cell Res. 192, 248–255. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

