Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene
- PMID: 15882914
- PMCID: PMC1950324
- DOI: 10.1016/j.molbrainres.2005.03.016
Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene
Abstract
The physiologic response to stress is highly dependent on the activation of corticotropin-releasing hormone (CRH) neurons by various neurotransmitters. A particularly rich innervation of hypophysiotropic CRH neurons has been detected by nerve fibers containing the neuropeptide PACAP, a potent activator of the cAMP-protein kinase A (PKA) system. Intracerebroventricular (icv) injections of PACAP also elevate steady-state CRH mRNA levels in the paraventricular nucleus (PVN), but it is not known whether PACAP effects can be associated with acute stress responses. Likewise, in cell culture studies, pharmacologic activation of the PKA system has stimulated CRH gene promoter activity through an identified cAMP response element (CRE); however, a direct link between PACAP and CRH promoter activity has not been established. In our present study, icv injection of 150 or 300 pmol PACAP resulted in robust phosphorylation of the transcription factor CREB in the majority of PVN CRH neurons at 15 to 30 min post-injection and induced nuclear Fos labeling at 90 min. Simultaneously, plasma corticosterone concentrations were elevated in PACAP-injected animals, and significant increases were observed in face washing, body grooming, rearing and wet-dog shakes behaviors. We investigated the effect of PACAP on human CRH promoter activity in alphaT3-1 cells, a PACAP-receptor expressing cell line. Cells were transiently transfected with a chloramphenicol acetyltransferase (CAT) reporter vector containing region - 663/+124 of the human CRH gene promoter then treated for with PACAP (100 nM) or with the adenylate cyclase activating agent, forskolin (2.5 muM). Both PACAP and forskolin significantly increased wild-type hCRH promoter activity relative to vehicle controls. The PACAP response was abolished in the CRE-mutant construct. Pretreatment of transfected cells with the PKA blocker, H-89, completely prevented both PACAP- and forskolin-induced increases in CRH promoter activity. Furthermore, CREB overexpression strongly enhanced PACAP-mediated stimulation of hCRH promoter activity, an effect which was also lost with mutation of the CRE. Thus, we demonstrate that icv PACAP administration to rats under non-stressed handling conditions leads to cellular, hormonal and behavioral responses recapitulating manifestations of the acute stress response. Both in vivo and in vitro data point to the importance of PACAP-mediated activation of the cAMP/PKA signaling pathway for stimulation of CRH gene transcription, likely via the CRE.
Figures


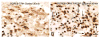


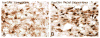


Similar articles
-
Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN).Regul Pept. 2005 May 15;128(1):33-41. doi: 10.1016/j.regpep.2004.12.023. Regul Pept. 2005. PMID: 15721485 Free PMC article.
-
Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus.Brain Res. 1997 Oct 31;773(1-2):190-6. doi: 10.1016/s0006-8993(97)01011-1. Brain Res. 1997. PMID: 9409720
-
Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress.Endocrinology. 1995 Sep;136(9):4116-24. doi: 10.1210/endo.136.9.7649120. Endocrinology. 1995. PMID: 7649120
-
Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus.Endocr J. 2009;56(3):335-44. doi: 10.1507/endocrj.k09e-075. Epub 2009 Apr 7. Endocr J. 2009. PMID: 19352056 Review.
-
Investigating the potential role of the pituitary adenylate cyclase-activating polypeptide (PACAP) in regulating the ubiquitin signaling pathway in poultry.Gen Comp Endocrinol. 2024 Sep 15;356:114577. doi: 10.1016/j.ygcen.2024.114577. Epub 2024 Jun 22. Gen Comp Endocrinol. 2024. PMID: 38914296 Review.
Cited by
-
Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies.Biol Psychiatry. 2015 Aug 1;78(3):167-77. doi: 10.1016/j.biopsych.2014.12.003. Epub 2014 Dec 9. Biol Psychiatry. 2015. PMID: 25636177 Free PMC article. Review.
-
Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) of the Bed Nucleus of the Stria Terminalis Mediates Heavy Alcohol Drinking in Mice.eNeuro. 2023 Dec 28;10(12):ENEURO.0424-23.2023. doi: 10.1523/ENEURO.0424-23.2023. Print 2023 Dec. eNeuro. 2023. PMID: 38053471 Free PMC article.
-
Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN).Regul Pept. 2005 May 15;128(1):33-41. doi: 10.1016/j.regpep.2004.12.023. Regul Pept. 2005. PMID: 15721485 Free PMC article.
-
Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience.Int J Mol Sci. 2021 Nov 12;22(22):12242. doi: 10.3390/ijms222212242. Int J Mol Sci. 2021. PMID: 34830130 Free PMC article. Review.
-
Vasoactive intestinal peptide is critical for circadian regulation of glucocorticoids.Neuroendocrinology. 2008;88(4):246-55. doi: 10.1159/000140676. Epub 2008 Jun 19. Neuroendocrinology. 2008. PMID: 18562786 Free PMC article.
References
-
- Adler GK, Smas CM, Fiandaca M, Frim DM, Majzoub JA. Regulated expression of the human corticotropin releasing hormone gene by cyclic AMP. Mol Cell Endocrinol. 1990:70. 165–174. - PubMed
-
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. - PubMed
-
- Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. - PubMed
-
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. - PubMed
-
- Ary M, Cox B, Lomax P. Dopaminergic mechanisms in precipitated withdrawal in morphine-dependent rats. J Pharmacol Exp Ther. 1977;200:271–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

