A cytomegaloviral protein reveals a dual role for STAT2 in IFN-{gamma} signaling and antiviral responses
- PMID: 15883169
- PMCID: PMC2212917
- DOI: 10.1084/jem.20041401
A cytomegaloviral protein reveals a dual role for STAT2 in IFN-{gamma} signaling and antiviral responses
Abstract
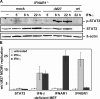
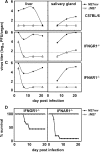
A mouse cytomegalovirus (MCMV) gene conferring interferon (IFN) resistance was identified. This gene, M27, encodes a 79-kD protein that selectively binds and down-regulates for signal transducer and activator of transcription (STAT)-2, but it has no effect on STAT1 activation and signaling. The absence of pM27 conferred MCMV susceptibility to type I IFNs (alpha/beta), but it had a much more dramatic effect on type II IFNs (gamma) in vitro and in vivo. A comparative analysis of M27(+) and M27(-) MCMV revealed that the antiviral efficiency of IFN-gamma was partially dependent on the synergistic action of type I IFNs that required STAT2. Moreover, STAT2 was directly activated by IFN-gamma. This effect required IFN receptor expression and was independent of type I IFNs. IFN-gamma induced increasing levels of tyrosine-phosphorylated STAT2 in M27(-) MCMV-infected cells that were essential for the antiviral potency of IFN-gamma. pM27 represents a new strategy for simultaneous evasions from types I and II IFNs, and it documents an unknown biological significance for STAT2 in antiviral IFN-gamma responses.
Figures


Similar articles
-
STAT2-Dependent Immune Responses Ensure Host Survival despite the Presence of a Potent Viral Antagonist.J Virol. 2018 Jun 29;92(14):e00296-18. doi: 10.1128/JVI.00296-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743368 Free PMC article.
-
The proximal tyrosines of the cytoplasmic domain of the beta chain of the type I interferon receptor are essential for signal transducer and activator of transcription (Stat) 2 activation. Evidence that two Stat2 sites are required to reach a threshold of interferon alpha-induced Stat2 tyrosine phosphorylation that allows normal formation of interferon-stimulated gene factor 3.J Biol Chem. 1999 Feb 12;274(7):4045-52. doi: 10.1074/jbc.274.7.4045. J Biol Chem. 1999. PMID: 9933596
-
Interferon-gamma inhibits interferon-alpha signalling in hepatic cells: evidence for the involvement of STAT1 induction and hyperexpression of STAT1 in chronic hepatitis C.Biochem J. 2004 Apr 1;379(Pt 1):199-208. doi: 10.1042/BJ20031495. Biochem J. 2004. PMID: 14690454 Free PMC article.
-
A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
-
The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses.Cytokine Growth Factor Rev. 2016 Jun;29:71-81. doi: 10.1016/j.cytogfr.2016.02.010. Epub 2016 Mar 18. Cytokine Growth Factor Rev. 2016. PMID: 27053489 Review.
Cited by
-
Interferon gamma protects neonatal neural stem/progenitor cells during measles virus infection of the brain.J Neuroinflammation. 2016 May 13;13(1):107. doi: 10.1186/s12974-016-0571-1. J Neuroinflammation. 2016. PMID: 27178303 Free PMC article.
-
Stepwise adaptation of murine cytomegalovirus to cells of a foreign host for identification of host range determinants.Med Microbiol Immunol. 2015 Jun;204(3):461-9. doi: 10.1007/s00430-015-0400-7. Epub 2015 Mar 19. Med Microbiol Immunol. 2015. PMID: 25788395
-
A Mass Spectrometry-Based Profiling of Interactomes of Viral DDB1- and Cullin Ubiquitin Ligase-Binding Proteins Reveals NF-κB Inhibitory Activity of the HIV-2-Encoded Vpx.Front Immunol. 2018 Dec 19;9:2978. doi: 10.3389/fimmu.2018.02978. eCollection 2018. Front Immunol. 2018. PMID: 30619335 Free PMC article.
-
STAT2-Dependent Immune Responses Ensure Host Survival despite the Presence of a Potent Viral Antagonist.J Virol. 2018 Jun 29;92(14):e00296-18. doi: 10.1128/JVI.00296-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743368 Free PMC article.
-
Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything.J Intern Med. 2010 May;267(5):483-501. doi: 10.1111/j.1365-2796.2010.02220.x. J Intern Med. 2010. PMID: 20433576 Free PMC article. Review.
References
-
- Darnell, J.E., Jr. 1997. STATs and gene regulation. Science. 277:1630–1635. - PubMed
-
- Stark, G.R., I.M. Kerr, B.R. Williams, R.H. Silverman, and R.D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227–264. - PubMed
-
- Bach, E.A., M. Aguet, and R.D. Schreiber. 1997. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15:563–591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

