Decay-accelerating factor modulates induction of T cell immunity
- PMID: 15883171
- PMCID: PMC2212927
- DOI: 10.1084/jem.20041967
Decay-accelerating factor modulates induction of T cell immunity
Abstract
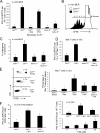
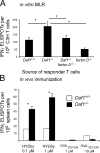
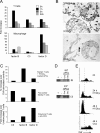
Decay-accelerating factor (Daf) dissociates C3/C5 convertases that assemble on host cells and thereby prevents complement activation on their surfaces. We demonstrate that during primary T cell activation, the absence of Daf on antigen-presenting cells (APCs) and on T cells enhances T cell proliferation and augments the induced frequency of effector cells. The effect is factor D- and, at least in part, C5-dependent, indicating that local alternative pathway activation is essential. We show that cognate T cell-APC interactions are accompanied by rapid production of alternative pathway components and down-regulation of Daf expression. The findings argue that local alternative pathway activation and surface Daf protein function respectively as a costimulator and a negative modulator of T cell immunity and explain previously reported observations linking complement to T cell function. The results could have broad therapeutic implications for disorders in which T cell immunity is important.
Figures


References
-
- Janeway, C., Jr., P. Travers, M. Walport, and M.J. Shlomchik. 2004. Innate immunity. In Immunobiology. Garland Publishing, New York. 37–100.
-
- Suresh, M., H. Molina, M.S. Salvato, D. Mastellos, J.D. Lambris, and M. Sandor. 2003. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J. Immunol. 170:788–794. - PubMed
-
- Pratt, J.R., S.A. Basheer, and S.H. Sacks. 2002. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat. Med. 8:582–587. - PubMed
-
- Nataf, S., S.L. Carroll, R.A. Wetsel, A.J. Szalai, and S.R. Barnum. 2000. Attenuation of experimental autoimmune demyelination in complement-deficient mice. J. Immunol. 165:5867–5873. - PubMed
-
- Kopf, M., B. Abel, A. Gallimore, M. Carroll, and M.F. Bachmann. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8:373–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

