Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response
- PMID: 15883172
- PMCID: PMC2212915
- DOI: 10.1084/jem.20042167
Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response
Abstract
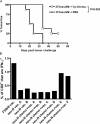
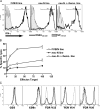
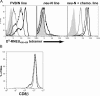
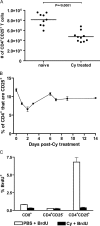
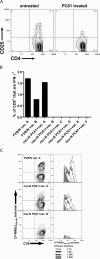
A major barrier to successful antitumor vaccination is tolerance of high-avidity T cells specific to tumor antigens. In keeping with this notion, HER-2/neu (neu)-targeted vaccines, which raise strong CD8(+) T cell responses to a dominant peptide (RNEU(420-429)) in WT FVB/N mice and protect them from a neu-expressing tumor challenge, fail to do so in MMTV-neu (neu-N) transgenic mice. However, treatment of neu-N mice with vaccine and cyclophosphamide-containing chemotherapy resulted in tumor protection in a proportion of mice. This effect was specifically abrogated by the transfer of neu-N-derived CD4(+)CD25(+) T cells. RNEU(420-429)-specific CD8(+) T cells were identified only in neu-N mice given vaccine and cyclophosphamide chemotherapy which rejected tumor challenge. Tetramer-binding studies demonstrated that cyclophosphamide pretreatment allowed the activation of high-avidity RNEU(420-429)-specific CD8(+) T cells comparable to those generated from vaccinated FVB/N mice. Cyclophosphamide seemed to inhibit regulatory T (T reg) cells by selectively depleting the cycling population of CD4(+)CD25(+) T cells in neu-N mice. These findings demonstrate that neu-N mice possess latent pools of high-avidity neu-specific CD8(+) T cells that can be recruited to produce an effective antitumor response if T reg cells are blocked or removed by using approaches such as administration of cyclophosphamide before vaccination.
Figures



References
-
- Sprent, J., and H. Kishimoto. 2002. The thymus and negative selection. Immunol. Rev. 185:126–135. - PubMed
-
- Walker, L.S., and A.K. Abbas. 2002. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2:11–19. - PubMed
-
- O'Garra, A., and P. Vieira. 2004. Regulatory T cells and mechanisms of immune system control. Nat. Med. 10:801–805. - PubMed
-
- Pardoll, D. 2003. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 21:807–839. - PubMed
-
- Disis, M.L., and M.A. Cheever. 1997. HER-2/neu protein: a target for antigen-specific immunotherapy of human cancer. Adv. Cancer Res. 71:343–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

