Regulation of phototransduction responsiveness and retinal degeneration by a phospholipase D-generated signaling lipid
- PMID: 15883198
- PMCID: PMC2171926
- DOI: 10.1083/jcb.200502122
Regulation of phototransduction responsiveness and retinal degeneration by a phospholipase D-generated signaling lipid
Abstract
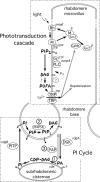
Drosophila melanogaster phototransduction proceeds via a phospholipase C (PLC)-triggered cascade of phosphatidylinositol (PI) lipid modifications, many steps of which remain undefined. We describe the involvement of the lipid phosphatidic acid and the enzyme that generates it, phospholipase D (Pld), in this process. Pld(null) flies exhibit decreased light sensitivity as well as a heightened susceptibility to retinal degeneration. Pld overexpression rescues flies lacking PLC from light-induced, metarhodopsin-mediated degeneration and restores visual signaling in flies lacking the PI transfer protein, which is a key player in the replenishment of the PI 4,5-bisphosphate (PIP2) substrate used by PLC to transduce light stimuli into neurological signals. Altogether, these findings suggest that Pld facilitates phototransduction by maintaining adequate levels of PIP2 and by protecting the visual system from metarhodopsin-induced, low light degeneration.
Figures






Similar articles
-
Phospholipase D activity couples plasma membrane endocytosis with retromer dependent recycling.Elife. 2016 Nov 16;5:e18515. doi: 10.7554/eLife.18515. Elife. 2016. PMID: 27848911 Free PMC article.
-
RdgBα reciprocally transfers PA and PI at ER-PM contact sites to maintain PI(4,5)P2 homoeostasis during phospholipase C signalling in Drosophila photoreceptors.Biochem Soc Trans. 2016 Feb;44(1):286-92. doi: 10.1042/BST20150228. Biochem Soc Trans. 2016. PMID: 26862217 Review.
-
Phototransduction and retinal degeneration in Drosophila.Pflugers Arch. 2007 Aug;454(5):821-47. doi: 10.1007/s00424-007-0251-1. Epub 2007 May 9. Pflugers Arch. 2007. PMID: 17487503 Review.
-
Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration.Science. 2003 Mar 14;299(5613):1740-3. doi: 10.1126/science.1080549. Science. 2003. PMID: 12637747
-
Substitution of a non-retinal phospholipase C in Drosophila phototransduction.Insect Mol Biol. 2003 Apr;12(2):147-53. doi: 10.1046/j.1365-2583.2003.00396.x. Insect Mol Biol. 2003. PMID: 12653936
Cited by
-
Drosophila photoreceptors and signaling mechanisms.Front Cell Neurosci. 2009 Jun 11;3:2. doi: 10.3389/neuro.03.002.2009. eCollection 2009. Front Cell Neurosci. 2009. PMID: 19623243 Free PMC article.
-
Phospholipase D: enzymology, functionality, and chemical modulation.Chem Rev. 2011 Oct 12;111(10):6064-119. doi: 10.1021/cr200296t. Epub 2011 Sep 22. Chem Rev. 2011. PMID: 21936578 Free PMC article. Review. No abstract available.
-
TRP channels and Ca2+ signaling.Cell Calcium. 2006 Sep;40(3):261-75. doi: 10.1016/j.ceca.2006.05.002. Epub 2006 Jun 27. Cell Calcium. 2006. PMID: 16806461 Free PMC article. Review.
-
Mass spectrometric analyses of phospholipids in the S334ter-3 rat model of retinal degeneration.Mol Vis. 2014 Nov 11;20:1605-11. eCollection 2014. Mol Vis. 2014. PMID: 25489232 Free PMC article.
-
Phospholipase D activity couples plasma membrane endocytosis with retromer dependent recycling.Elife. 2016 Nov 16;5:e18515. doi: 10.7554/eLife.18515. Elife. 2016. PMID: 27848911 Free PMC article.
References
-
- Agam, K., S. Frechter, and B. Minke. 2004. Activation of the Drosophila TRP and TRPL channels requires both Ca2+ and protein dephosphorylation. Cell Calcium. 35:87–105. - PubMed
-
- Alloway, P.G., L. Howard, and P.J. Dolph. 2000. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 28:129–138. - PubMed
-
- Boesze-Battaglia, K., O.P. Lamba, A.A. Napoli Jr., S. Sinha, and Y. Guo. 1998. Fusion between retinal rod outer segment membranes and model membranes: a role for photoreceptor peripherin/rds. Biochemistry. 37:9477–9487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

