Structural properties of Abeta protofibrils stabilized by a small molecule
- PMID: 15883377
- PMCID: PMC1091746
- DOI: 10.1073/pnas.0408582102
Structural properties of Abeta protofibrils stabilized by a small molecule
Abstract
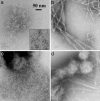
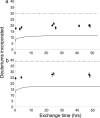
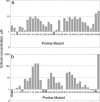
Metastable oligomeric and protofibrillar forms of amyloidogenic proteins have been implicated as on-pathway assembly intermediates in amyloid formation and as the major toxic species in a number of amyloid diseases including Alzheimer's disease. We describe here a chemical biology approach to structural analysis of Abeta protofibrils. Library screening yielded several molecules that stimulate Abeta aggregation. One of these compounds, calmidazolium chloride (CLC), rapidly and efficiently converts Abeta(1-40) monomers into clusters of protofibrils. As monitored by electron microscopy, these protofibrils persist for days when incubated in PBS at 37 degrees C, with a slow transition to fibrillar structures apparent only after several weeks. Like normal protofibrils, the CLC-Abeta aggregates exhibit a low thioflavin T response. Like Abeta fibrils, the clustered protofibrils bind the anti-amyloid Ab WO1. The CLC-Abeta aggregates exhibit the same protection from hydrogen-deuterium exchange as do protofibrils isolated from a spontaneous Abeta fibril formation reaction: approximately 12 of the 39 Abeta(1-40) backbone amide protons are protected from exchange in the protofibril, compared with approximately twice that number in amyloid fibrils. Scanning proline mutagenesis analysis shows that the Abeta molecule in these protofibrillar assemblies exhibits the same flexible N and C termini as do mature amyloid fibrils. The major difference in Abeta conformation between fibrils and protofibrils is added structural definition in the 22-29 segment in the fibril. Besides aiding structural analysis, compounds capable of facilitating oligomer and protofibril formation might have therapeutic potential, if they act to sequester Abeta in a form and/or location that cannot engage the toxic pathway.
Figures



Similar articles
-
Structural differences in Abeta amyloid protofibrils and fibrils mapped by hydrogen exchange--mass spectrometry with on-line proteolytic fragmentation.J Mol Biol. 2006 Aug 25;361(4):785-95. doi: 10.1016/j.jmb.2006.06.066. Epub 2006 Jul 12. J Mol Biol. 2006. PMID: 16875699
-
Abeta protofibrils possess a stable core structure resistant to hydrogen exchange.Biochemistry. 2003 Dec 9;42(48):14092-8. doi: 10.1021/bi0357816. Biochemistry. 2003. PMID: 14640676
-
Growth of beta-amyloid(1-40) protofibrils by monomer elongation and lateral association. Characterization of distinct products by light scattering and atomic force microscopy.Biochemistry. 2002 May 14;41(19):6115-27. doi: 10.1021/bi015985r. Biochemistry. 2002. PMID: 11994007
-
Plasticity of amyloid fibrils.Biochemistry. 2007 Jan 9;46(1):1-10. doi: 10.1021/bi0620959. Biochemistry. 2007. PMID: 17198370 Free PMC article. Review.
-
Electrochemistry of Alzheimer Disease Amyloid Beta Peptides.Curr Med Chem. 2018;25(33):4066-4083. doi: 10.2174/0929867325666180214112536. Curr Med Chem. 2018. PMID: 29446720 Review.
Cited by
-
Unveiling the Potential of Polyphenols as Anti-Amyloid Molecules in Alzheimer's Disease.Curr Neuropharmacol. 2023;21(4):787-807. doi: 10.2174/1570159X20666221010113812. Curr Neuropharmacol. 2023. PMID: 36221865 Free PMC article. Review.
-
C-Terminal Threonine Reduces Aβ43 Amyloidogenicity Compared with Aβ42.J Mol Biol. 2016 Jan 29;428(2 Pt A):274-291. doi: 10.1016/j.jmb.2015.06.008. Epub 2015 Jun 26. J Mol Biol. 2016. PMID: 26122432 Free PMC article.
-
Halogenation generates effective modulators of amyloid-Beta aggregation and neurotoxicity.PLoS One. 2013;8(2):e57288. doi: 10.1371/journal.pone.0057288. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23468958 Free PMC article.
-
Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils.J Neurosci. 2010 Oct 27;30(43):14411-9. doi: 10.1523/JNEUROSCI.3537-10.2010. J Neurosci. 2010. PMID: 20980598 Free PMC article.
-
Constraints on the Structure of Fibrils Formed by a Racemic Mixture of Amyloid-β Peptides from Solid-State NMR, Electron Microscopy, and Theory.J Am Chem Soc. 2021 Aug 25;143(33):13299-13313. doi: 10.1021/jacs.1c06339. Epub 2021 Aug 10. J Am Chem Soc. 2021. PMID: 34375097 Free PMC article.
References
-
- Walsh, D. M., Hartley, D. M., Kusumoto, Y., Fezoui, Y., Condron, M. M., Lomakin, A., Benedek, G. B., Selkoe, D. J. & Teplow, D. B. (1999) J. Biol. Chem. 274, 25945-25952. - PubMed
-
- Harper, J. D., Wong, S. S., Lieber, C. M. & Lansbury, P. T., Jr. (1999) Biochemistry 38, 8972-8980. - PubMed
-
- Kirkitadze, M. D., Bitan, G. & Teplow, D. B. (2002) J. Neurosci. Res. 69, 567-577. - PubMed
-
- Caughey, B. & Lansbury, P. T. (2003) Annu. Rev. Neurosci. 26, 267-298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

