Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum
- PMID: 15883387
- PMCID: PMC1140406
- DOI: 10.1073/pnas.0408531102
Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum
Abstract
Corticotropin-releasing factor (CRF) and the closely related family of neuropeptides urocortins (Ucns) are ancient paracrine-signaling peptides secreted in both the central and peripheral neural circuits. CRF and Ucns released from the CNS (central) regulate a plethora of physiological processes that include food intake, inflammation, and bowel motility and permeability. In the gastrointestinal tract, CRF actions are largely proinflammatory, whereas the effects of the Ucn subtypes can be either pro- or antiinflammatory. Central (intracerebroventricular) or peripheral (i.p.) administration of CRF or Ucns inhibits gastric emptying and promotes colonic motility. To ascertain the role of peripherally expressed CRF and UcnII in gastrointestinal inflammation and motility, we generated ileum-specific phenotypic knockouts of these peptides by using RNA interference. Long dsRNA effectively silenced basal expression of CRF and UcnII in ileum. Control dsRNA or saline treatment did not affect CRF or UcnII expression. In an experimental model of toxin-induced intestinal inflammation, inhibition of CRF ablated the inflammatory response (measured by epithelial damage, mucosal edema, and neutrophil infiltration). UcnII dsRNA treatment did not alter the inflammatory response to toxin. Furthermore, ileal motility was increased after site-specific inhibition of both CRF and UcnII. Thus, we demonstrate that ileal-specific CRF promotes inflammation and both CRF and UcnII modulate bowel motility.
Figures
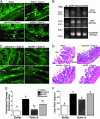
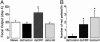
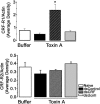

Comment in
-
The stressed gut: contributions of intestinal stress peptides to inflammation and motility.Proc Natl Acad Sci U S A. 2005 May 24;102(21):7409-10. doi: 10.1073/pnas.0503092102. Epub 2005 May 17. Proc Natl Acad Sci U S A. 2005. PMID: 15899972 Free PMC article. No abstract available.
Similar articles
-
Endogenous expression and in vitro study of CRF-related peptides and CRF receptors in the rat gastric antrum.Peptides. 2006 Jun;27(6):1464-75. doi: 10.1016/j.peptides.2005.10.023. Epub 2005 Dec 6. Peptides. 2006. PMID: 16337313
-
Urocortins and the regulation of gastrointestinal motor function and visceral pain.Peptides. 2004 Oct;25(10):1733-44. doi: 10.1016/j.peptides.2004.05.025. Peptides. 2004. PMID: 15476940 Review.
-
Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine.Am J Physiol Gastrointest Liver Physiol. 2005 May;288(5):G1091-103. doi: 10.1152/ajpgi.00302.2004. Epub 2005 Jan 6. Am J Physiol Gastrointest Liver Physiol. 2005. PMID: 15637181
-
Expression of corticotropin-releasing factor and urocortins in the normal and Schistosoma mansoni-infected mouse ileum.Cell Tissue Res. 2015 Feb;359(2):453-463. doi: 10.1007/s00441-014-2012-8. Epub 2014 Oct 31. Cell Tissue Res. 2015. PMID: 25358399
-
Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function.Neurogastroenterol Motil. 2004 Apr;16 Suppl 1:137-42. doi: 10.1111/j.1743-3150.2004.00490.x. Neurogastroenterol Motil. 2004. PMID: 15066020 Review.
Cited by
-
Local injection of dsRNA targeting calcitonin receptor-like receptor (CLR) ameliorates Clostridium difficile toxin A-induced ileitis.Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):731-6. doi: 10.1073/pnas.1219733110. Epub 2012 Dec 24. Proc Natl Acad Sci U S A. 2013. PMID: 23267070 Free PMC article.
-
Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability.J Allergy Clin Immunol. 2019 May;143(5):1865-1877.e4. doi: 10.1016/j.jaci.2018.08.053. Epub 2018 Nov 12. J Allergy Clin Immunol. 2019. PMID: 30439403 Free PMC article.
-
Inhibition of corticotropin-releasing hormone receptor 1 and activation of receptor 2 protect against colonic injury and promote epithelium repair.Sci Rep. 2017 May 11;7:46616. doi: 10.1038/srep46616. Sci Rep. 2017. PMID: 28492284 Free PMC article.
-
Urocortin 3 expression at baseline and during inflammation in the colon: corticotropin releasing factor receptors cross-talk.Peptides. 2014 Apr;54:58-66. doi: 10.1016/j.peptides.2014.01.007. Epub 2014 Jan 22. Peptides. 2014. PMID: 24462512 Free PMC article.
-
Endothelin-converting enzyme-1 actions determine differential trafficking and signaling of corticotropin-releasing factor receptor 1 at high agonist concentrations.Mol Endocrinol. 2012 Apr;26(4):681-95. doi: 10.1210/me.2011-1361. Epub 2012 Feb 9. Mol Endocrinol. 2012. PMID: 22322595 Free PMC article.
References
-
- Vaughan, J., Donaldson, C., Bittencourt, J., Perrin, M. H., Lewis, K., Sutton, S., Chan, R., Turnbull, A. V., Lovejoy, D., Rivier, C., et al. (1995) Nature 378, 287–292. - PubMed
-
- Grigoriadis, D. E., Lovenberg, T. W., Chalmers, D. T., Liaw, C. & De Souze, E. B. (1996) Ann. N.Y. Acad. Sci. 780, 60–80. - PubMed
-
- Swanson, L. W., Sawchenko, P. E., Rivier, J. & Vale, W. W. (1983) Neuroendocrinology 36, 165–186. - PubMed

