The vertebrate social behavior network: evolutionary themes and variations
- PMID: 15885690
- PMCID: PMC2570781
- DOI: 10.1016/j.yhbeh.2005.02.003
The vertebrate social behavior network: evolutionary themes and variations
Abstract
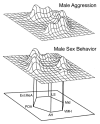
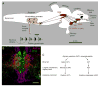
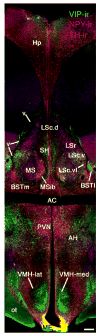
Based on a wide variety of data, it is now clear that birds and teleost (bony) fish possess a core "social behavior network" within the basal forebrain and midbrain that is homologous to the social behavior network of mammals. The nodes of this network are reciprocally connected, contain receptors for sex steroid hormones, and are involved in multiple forms of social behavior. Other hodological features and neuropeptide distributions are likewise very similar across taxa. This evolutionary conservation represents a boon for experiments on phenotypic behavioral variation, as the extraordinary social diversity of teleost fish and songbirds can now be used to generate broadly relevant insights into issues of brain function that are not particularly tractable in other vertebrate groups. Two such lines of research are presented here, each of which addresses functional variation within the network as it relates to divergent patterns of social behavior. In the first set of experiments, we have used a sexually polymorphic fish to demonstrate that natural selection can operate independently on hypothalamic neuroendocrine functions that are relevant for (1) gonadal regulation and (2) sex-typical behavioral modulation. In the second set of experiments, we have exploited the diversity of avian social organizations and ecologies to isolate species-typical group size as a quasi-independent variable. These experiments have shown that specific areas and peptidergic components of the social behavior network possess functional properties that evolve in parallel with divergence and convergence in sociality.
Figures
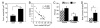
References
-
- Absil P, Braquenier JB, Balthazart J, Ball GF. Effects of lesions of nucleus taeniae on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Brain Behav Evol. 2002;60:13–35. - PubMed
-
- Acher R. Endocrinology. American Physiological Society; Washington, D.C.: 1972. Chemistry of the neurohypophysial hormones: an example of molecular evolution; pp. 119–130.
-
- Aste N, Balthazart J, Absil P, Grossmann R, Mulhbauer E, Viglietti-Panzica C, Panzica GC. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica) J Comp Neurol. 1998;396:141–157. - PubMed
-
- Aste N, Viglietti-Panzica C, Balthazart J, Panzica GC. Testosterone modulation of peptidergic pathways in the septo-preoptic region of male Japanese quail. Poult Avian Biol Rev. 1997;8:77–93.
-
- Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol. 2004;475:426–461. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

