fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses
- PMID: 15886103
- PMCID: PMC2790416
- DOI: 10.1016/j.cub.2005.03.047
fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses
Abstract
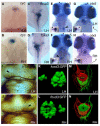
Asymmetries in CNS neuroanatomy are assumed to underlie the widespread cognitive and behavioral asymmetries in vertebrates. Studies in humans have shown that the laterality of some cognitive asymmetries is independent of the laterality of the viscera; discrete mechanisms may therefore regulate visceral and neural lateralization. However, through analysis of visceral, neuroanatomical, and behavioral asymmetries in the frequent-situs-inversus (fsi) line of zebrafish, we show that the principal left-right body asymmetries are coupled to certain brain asymmetries and lateralized behaviors. fsi fish with asymmetry defects show concordant reversal of heart, gut, and neuroanatomical asymmetries in the diencephalon. Moreover, the neuroanatomical reversals in reversed fsi fish correlate with reversal of some behavioral responses in both fry and adult fsi fish. Surprisingly, two behavioral asymmetries do not reverse, suggesting that at least two separable mechanisms must influence functional lateralization in the CNS. Partial reversal of CNS asymmetries may generate new behavioral phenotypes; supporting this idea, reversed fsi fry differ markedly from their normally lateralized siblings in their behavioral response to a novel visual feature. Revealing a link between visceral and brain asymmetry and lateralized behavior, our studies help to explain the complexity of the relationship between the lateralities of visceral and neural asymmetries.
Figures



Comment in
-
Brain asymmetry: switching from left to right.Curr Biol. 2005 May 10;15(9):R343-5. doi: 10.1016/j.cub.2005.04.026. Curr Biol. 2005. PMID: 15886094 Review.
References
-
- Cooke J. Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biol. Rev. Camb. Philos. Soc. 2004;79:377–407. - PubMed
-
- Malashichev YB, Wassersug RJ. Left and right in the amphibian world: Which way to develop and where to turn? Bioessays. 2004;26:512–522. - PubMed
-
- Vallortigara G, Rogers LJ. Behavioural and Brain Sciences. Cambridge University Press; Cambridge: 2004. Survival with an Asymmetrical Brain: Advantages and Disadvantages of Cerebral Lateralization. - PubMed
-
- Palmer AR. Symmetry breaking and the evolution of development. Science. 2004;306:828–833. - PubMed
-
- Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567–3579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

