Myosin VIIA defects, which underlie the Usher 1B syndrome in humans, lead to deafness in Drosophila
- PMID: 15886106
- PMCID: PMC1808204
- DOI: 10.1016/j.cub.2005.03.050
Myosin VIIA defects, which underlie the Usher 1B syndrome in humans, lead to deafness in Drosophila
Abstract
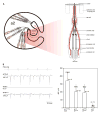
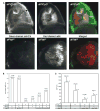
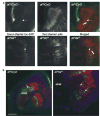
In vertebrates, auditory and vestibular transduction occurs on apical projections (stereocilia) of specialized cells (hair cells). Mutations in myosin VIIA (myoVIIA), an unconventional myosin, lead to deafness and balance anomalies in humans, mice, and zebrafish; individuals are deaf, and stereocilia are disorganized. The exact mechanism through which myoVIIA mutations result in these inner-ear anomalies is unknown. Proposed inner-ear functions for myoVIIA include anchoring transduction channels to the stereocilia membrane, trafficking stereocilia linking components, and anchoring hair cells by associating with adherens junctions. The Drosophila myoVIIA homolog is crinkled (ck). The Drosophila auditory organ, Johnston's organ (JO), is developmentally and functionally related to the vertebrate inner ear. Both derive from modified epithelial cells specified by atonal and spalt homolog expression, and both transduce acoustic mechanical energy (and references therein). Here, we show that loss of ck/myoVIIA function leads to complete deafness in Drosophila by disrupting the integrity of the scolopidia that transduce auditory signals. We demonstrate that ck/myoVIIA functions to organize the auditory organ, that it is functionally required in neuronal and support cells, that it is not required for TRPV channel localization, and that it is not essential for scolopidial-cell-junction integrity.
Figures

References
-
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. - PubMed
-
- Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, Beisel KW, Steel KP, Brown SD. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. - PubMed
-
- Ernest S, Rauch GJ, Haffter P, Geisler R, Petit C, Nicolson T. Mariner is defective in myosin VIIA: A zebra-fish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. - PubMed
-
- Self T, Mahoney M, Fleming J, Walsh J, Brown SDM, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

