GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow
- PMID: 15886326
- PMCID: PMC1895202
- DOI: 10.1182/blood-2004-11-4434
GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow
Abstract
The roles of the 2 major platelet-collagen receptors, glycoprotein VI (GPVI) and integrin alpha2beta1, have been intensely investigated using a variety of methods over the past decade. In the present study, we have used pharmacologic and genetic approaches to study human and mouse platelet adhesion to collagen under flow conditions. Our studies demonstrate that both GPVI and integrin alpha2beta1 play significant roles for platelet adhesion to collagen under flow and that the loss of both receptors completely ablates this response. Intracellular signaling mediated by the cytoplasmic adaptor Src homology 2 domain-containing leukocyte protein of 76 kDa (SLP-76) but not by the transmembrane adaptor linker for activation of T cells (LAT) is critical for platelet adhesion to collagen under flow. In addition, reduced GPVI receptor density results in severe defects in platelet adhesion to collagen under flow. Defective adhesion to collagen under flow is associated with prolonged tail-bleeding times in mice lacking one or both collagen receptors. These studies establish platelet-collagen responses under physiologic flow as the consequence of a close partnership between 2 structurally distinct receptors and suggest that both receptors play significant hemostatic roles in vivo.
Figures

 ). The results shown were determined by image analysis of the phase-contrast images and are the mean ± SEM (n = 5). Statistically significant inhibition was seen at each shear rate for Ha1/29 (P < .05) and EDTA (P < .05). (B) Representative phase-contrast images captured at 400, 700, 1000, and 1300 s–1 after 4 minutes of perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
). The results shown were determined by image analysis of the phase-contrast images and are the mean ± SEM (n = 5). Statistically significant inhibition was seen at each shear rate for Ha1/29 (P < .05) and EDTA (P < .05). (B) Representative phase-contrast images captured at 400, 700, 1000, and 1300 s–1 after 4 minutes of perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.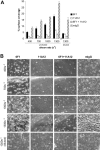
 ), or 20 μg/mL mouse IgG (mIgG;
), or 20 μg/mL mouse IgG (mIgG;  ) for at least 30 minutes prior to being perfused over a collagen-coated surface (“undiluted”). To facilitate comparison with the mouse platelet studies shown in Figure 1, this experiment was also performed using human blood diluted with an equal volume of Tyrode buffer (“diluted”). The results shown are the mean ± SEM (n = 4-5). Statistically significant inhibition was seen with all treatments (P < .05) at all shear rates with undiluted blood, and at 1300 s–1 with diluted blood. (B) Representative phase-contrast images after 4 minutes of perfusion of heparinized whole human blood over an immobilized collagen surface. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
) for at least 30 minutes prior to being perfused over a collagen-coated surface (“undiluted”). To facilitate comparison with the mouse platelet studies shown in Figure 1, this experiment was also performed using human blood diluted with an equal volume of Tyrode buffer (“diluted”). The results shown are the mean ± SEM (n = 4-5). Statistically significant inhibition was seen with all treatments (P < .05) at all shear rates with undiluted blood, and at 1300 s–1 with diluted blood. (B) Representative phase-contrast images after 4 minutes of perfusion of heparinized whole human blood over an immobilized collagen surface. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.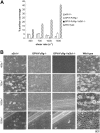
 ), GPVI-FcRγ–/– (□), GPVI-FcRγ–/–; α2β1–/– (▪), and wild-type (
), GPVI-FcRγ–/– (□), GPVI-FcRγ–/–; α2β1–/– (▪), and wild-type ( ) after flow. The results shown are the mean ± SEM (n = 6-11). Statistical significance (P < .01) was seen between the knockouts and the control at and between 400 and 1300 s–1. (B) Representative phase-contrast images after perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
) after flow. The results shown are the mean ± SEM (n = 6-11). Statistical significance (P < .01) was seen between the knockouts and the control at and between 400 and 1300 s–1. (B) Representative phase-contrast images after perfusion. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.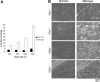
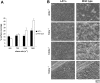
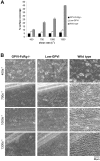
 ) platelets. The results shown are the mean ± SEM after analysis of the phase-contrast images (n = 5). Statistical significance was seen between GPVI-FcRγ–/–, low-GPVI, and wild-type at and between 400 s–1 and 1300 s–1 (P < .01). (B) Representative phase-contrast images after flow. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
) platelets. The results shown are the mean ± SEM after analysis of the phase-contrast images (n = 5). Statistical significance was seen between GPVI-FcRγ–/–, low-GPVI, and wild-type at and between 400 s–1 and 1300 s–1 (P < .01). (B) Representative phase-contrast images after flow. The lines seen in the upper left portions of some panels are visual artifact and do not arise due to abnormalities in the collagen-coated surface.
References
-
- Moroi M, Jung SM, Shinmyozu K, Tomiyama Y, Ordinas A, Diaz-Ricart M. Analysis of platelet adhesion to a collagen-coated surface under flow conditions: the involvement of glycoprotein VI in the platelet adhesion. Blood. 1996;88: 2081-2092. - PubMed
-
- Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94: 657-666. - PubMed
-
- Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88: 1525-1541. - PubMed
-
- Conti CR, Mehta JL. Acute myocardial ischemia: role of atherosclerosis, thrombosis, platelet activation, coronary vasospasm, and altered arachidonic acid metabolism. Circulation. 1987;75: V84-V95. - PubMed
-
- Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992;326: 310-318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

