Molecular preservation in Late Cretaceous sauropod dinosaur eggshells
- PMID: 15888409
- PMCID: PMC1599869
- DOI: 10.1098/rspb.2004.2876
Molecular preservation in Late Cretaceous sauropod dinosaur eggshells
Abstract
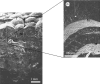
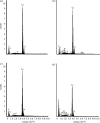
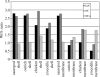
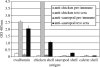
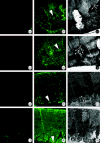
Exceptionally preserved sauropod eggshells discovered in Upper Cretaceous (Campanian) deposits in Patagonia, Argentina, contain skeletal remains and soft tissues of embryonic Titanosaurid dinosaurs. To preserve these labile embryonic remains, the rate of mineral precipitation must have superseded post-mortem degradative processes, resulting in virtually instantaneous mineralization of soft tissues. If so, mineralization may also have been rapid enough to retain fragments of original biomolecules in these specimens. To investigate preservation of biomolecular compounds in these well-preserved sauropod dinosaur eggshells, we applied multiple analytical techniques. Results demonstrate organic compounds and antigenic structures similar to those found in extant eggshells.
Figures



References
-
- Arias J.L, Fernandez M.S. Role of extracellular matrix molecules in shell formation and structure. World Poult. J. 2001;57:349–355.
-
- Armstrong W.G, Halstead L.B, Reed F.B, Wood L. Fossil proteins in vertebrate calcified tissues. Phil. Trans. R. Soc. B. 1983;301:301–343.
-
- Bada J.L. Amino acid racemization dating of fossils. Annu. Rev. Earth Planet. Sci. 1985;13:241–268.
-
- Baird R.F, Rowley M.J. Preservation of avian collagen in Australian quaternary cave deposits. Paleontology. 1990;33:442–451.
-
- Borja C, Garcia-Pacheco M, Olivares E.G, Scheuenstuhl G, Lowenstein J.M. Immunospecificity of albumin detected in 1.6 million-year-old fossils from Venta Micena in Orce, Granada, Spain. Am. J. Phys. Anthropol. 1997;103:433–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

