Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates
- PMID: 15888538
- PMCID: PMC1939694
- DOI: 10.1093/brain/awh526
Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates
Abstract
We report seven patients, six from a single institution, who developed subacute limbic encephalitis initially considered of uncertain aetiology. Four patients presented with symptoms of hippocampal dysfunction (i.e. severe short-term memory loss) and three with extensive limbic dysfunction (i.e. confusion, seizures and suspected psychosis). Brain MRI and [(18)F]fluorodeoxyglucose (FDG)-PET complemented each other but did not overlap in 50% of the patients. Combining both tests, all patients had temporal lobe abnormalities, five with additional areas involved. In one patient, FDG hyperactivity in the brainstem that was normal on MRI correlated with central hypoventilation; in another case, hyperactivity in the cerebellum anticipated ataxia. All patients had abnormal CSF: six pleocytosis, six had increased protein concentration, and three of five examined had oligoclonal bands. A tumour was identified and removed in four patients (mediastinal teratoma, thymoma, thymic carcinoma and thyroid cancer) and not treated in one (ovarian teratoma). An immunohistochemical technique that facilitates the detection of antibodies to cell surface or synaptic proteins demonstrated that six patients had antibodies to the neuropil of hippocampus or cerebellum, and one to intraneuronal antigens. Only one of the neuropil antibodies corresponded to voltage-gated potassium channel (VGKC) antibodies; the other five (two with identical specificity) reacted with antigens concentrated in areas of high dendritic density or synaptic-enriched regions of the hippocampus or cerebellum. Preliminary characterization of these antigens indicates that they are diverse and expressed on the neuronal cell membrane and dendrites; they do not co-localize with VGKCs, but partially co-localize with spinophilin. A target autoantigen in one of the patients co-localizes with a cell surface protein involved in hippocampal dendritic development. All patients except the one with antibodies to intracellular antigens had dramatic clinical and neuroimaging responses to immunotherapy or tumour resection; two patients had neurological relapse and improved with immunotherapy. Overall, the phenotype associated with the novel neuropil antibodies includes dominant behavioural and psychiatric symptoms and seizures that often interfere with the evaluation of cognition and memory, and brain MRI or FDG-PET abnormalities less frequently restricted to the medial temporal lobes than in patients with classical paraneoplastic or VGKC antibodies. When compared with patients with VGKC antibodies, patients with these novel antibodies are more likely to have CSF inflammatory abnormalities and systemic tumours (teratoma and thymoma), and they do not develop SIADH-like hyponatraemia. Although most autoantigens await characterization, all share intense expression by the neuropil of hippocampus, with patterns of immunolabelling characteristic enough to suggest the diagnosis of these disorders and predict response to treatment.
Figures
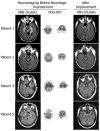
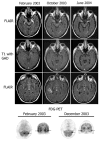
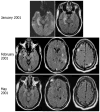


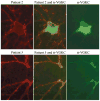

Comment in
-
A new cause of limbic encephalopathy.Brain. 2005 Aug;128(Pt 8):1745-6. doi: 10.1093/brain/awh592. Brain. 2005. PMID: 16030181 No abstract available.
References
-
- Alamowitch S, Graus F, Uchuya M, Reñé R, Bescansa E, Delattre JY. Limbic encephalitis and small cell lung cancer—clinical and immunological features. Brain. 1997;120:923–8. - PubMed
-
- Bataller L, Dalmau J. Paraneoplastic disorders of the central nervous system: update on diagnostic criteria and treatment. Semin Neurol. 2004;24:461–71. - PubMed
-
- Bataller L, Rosenfeld MR, Graus F, Vilchez JJ, Cheung NK, Dalmau J. Autoantigen diversity in the opsoclonus–myoclonus syndrome. Ann Neurol. 2003;53:347–53. - PubMed
-
- Benyahia B, Liblau R, Merle-Beral H, Tourani JM, Dalmau J, Delattre JY. Cell-mediated autoimmunity in paraneoplastic neurological syndromes with anti-Hu antibodies. Ann Neurol. 1999;45:162–7. - PubMed
-
- Bernal F, Graus F, Pifarre A, Saiz A, Benyahia B, Ribalta T. Immunohistochemical analysis of anti-Hu-associated paraneoplastic encephalomyelitis. Acta Neuropathol. 2002;103:509–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

