Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease
- PMID: 15888786
- PMCID: PMC1774527
- DOI: 10.1136/gut.2004.046516
Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease
Abstract
Background and aims: A characteristic feature of Crohn's disease (CD) is mesenteric adipose tissue hypertrophy. Mesenteric adipocytes or specific proteins secreted by them may play a role in the pathogenesis of CD. We recently identified adiponectin as an adipocyte specific protein with anti-inflammatory properties. Here we report on expression of adiponectin in mesenteric adipose tissue of CD patients.
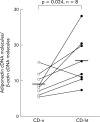
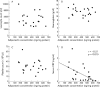
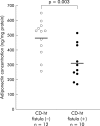
Methods and results: Mesenteric adipose tissue specimens were obtained from patients with CD (n = 22), ulcerative colitis (UC) (n = 8) and, for controls, colon carcinoma patients (n = 28) who underwent intestinal resection. Adiponectin concentrations were determined by enzyme linked immunosorbent assay, and adiponectin mRNA levels were determined by real time quantitative reverse transcription-polymerase chain reaction. Tissue concentrations and release of adiponectin were significantly increased in hypertrophied mesenteric adipose tissue of CD patients compared with normal mesenteric adipose tissue of CD patients (p = 0.002, p = 0.040, respectively), UC patients (p = 0.002, p = 0.003), and controls (p<0.0001, p<0.0001). Adiponectin mRNA levels were significantly higher in hypertrophied mesenteric adipose tissue of CD patients than in paired normal mesenteric adipose tissue from the same subjects (p = 0.024). Adiponectin concentrations in hypertrophied mesenteric adipose tissue of CD patients with an internal fistula were significantly lower than those of CD patients without an internal fistula (p = 0.003).
Conclusions: Our results suggest that adipocytes in hypertrophied mesenteric adipose tissue produce and secrete significant amounts of adiponectin, which could be involved in the regulation of intestinal inflammation associated with CD.
Figures





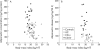
Comment in
-
Creeping fat in Crohn's disease: travelling in a creeper lane of research?Gut. 2005 Jun;54(6):742-4. doi: 10.1136/gut.2004.061531. Gut. 2005. PMID: 15888774 Free PMC article. No abstract available.
References
-
- Sheehan AL, Warren BF, Gear MW, et al. Fat-wrapping in Crohn’s disease: pathological basis and relevance to surgical practice. Br J Surg 1992;79:955–8. - PubMed
-
- Smedh K, Olaison G, Nystrom PO, et al. Intraoperative enteroscopy in Crohn’s disease. Br J Surg 1993;80:897–900. - PubMed
-
- Weakley FL, Turnbull RB. Recognition of regional ileitis in the operating room. Dis Colon Rectum 1971;14:17–23. - PubMed
-
- Fazio VW, Jones IT. Standard surgical treatment of Crohn’s disease of the small intestine and ileocolitis. In: Lee ECG, Nolan DJ, eds. Surgery of inflammatory bowel disorders. Edinburgh: Churchill Livingstone, 1987:147–56.
-
- Borley NR, Mortensen NJ, Jewell DP, et al. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn’s disease: evidence for a possible causative link. J Pathol 2000;190:196–202. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
