Oncogenic transcription factor Evi1 regulates hematopoietic stem cell proliferation through GATA-2 expression
- PMID: 15889140
- PMCID: PMC1142611
- DOI: 10.1038/sj.emboj.7600679
Oncogenic transcription factor Evi1 regulates hematopoietic stem cell proliferation through GATA-2 expression
Abstract
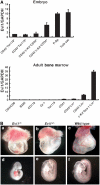
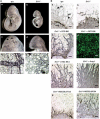
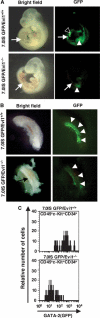
The ecotropic viral integration site-1 (Evi1) is an oncogenic transcription factor in murine and human myeloid leukemia. We herein show that Evi1 is predominantly expressed in hematopoietic stem cells (HSCs) in embryos and adult bone marrows, suggesting a physiological role of Evi1 in HSCs. We therefore investigate the role and authentic target genes of Evi1 in hematopoiesis using Evi1-/- mice, which die at embryonic day 10.5. HSCs in Evi1-/- embryos are markedly decreased in numbers in vivo with defective self-renewing proliferation and repopulating capacity. Notably, expression rate of GATA-2 mRNA, which is essential for proliferation of definitive HSCs, is profoundly reduced in HSCs of Evi1-/- embryos. Restoration of the Evi1 or GATA-2 expression in Evi1-/- HSCs could prevent the failure of in vitro maintenance and proliferation of HSC through upregulation of GATA-2 expression. An analysis of the GATA-2 promoter region revealed that Evi1 directly binds to GATA-2 promoter as an enhancer. Our results reveal that GATA-2 is presumably one of critical targets for Evi1 and that transcription factors regulate the HSC pool hierarchically.
Figures




Similar articles
-
Mice carrying a hypomorphic Evi1 allele are embryonic viable but exhibit severe congenital heart defects.PLoS One. 2014 Feb 27;9(2):e89397. doi: 10.1371/journal.pone.0089397. eCollection 2014. PLoS One. 2014. PMID: 24586749 Free PMC article.
-
Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity.J Exp Med. 2011 Nov 21;208(12):2403-16. doi: 10.1084/jem.20110447. Epub 2011 Nov 14. J Exp Med. 2011. PMID: 22084405 Free PMC article.
-
Survivin modulates genes with divergent molecular functions and regulates proliferation of hematopoietic stem cells through Evi-1.Leukemia. 2015 Feb;29(2):433-40. doi: 10.1038/leu.2014.183. Epub 2014 Jun 6. Leukemia. 2015. PMID: 24903482 Free PMC article.
-
Pattern of expression and their clinical implications of the GATA family, stem cell leukemia gene, and EVI1 in leukemia and myelodysplastic syndromes.Leuk Lymphoma. 1996 Nov;23(5-6):431-6. doi: 10.3109/10428199609054850. Leuk Lymphoma. 1996. PMID: 9031072 Review.
-
[The role and regulation of EVI1 in normal hematopoiesis and hematopoietic malignancies].Rinsho Ketsueki. 2024;65(9):954-960. doi: 10.11406/rinketsu.65.954. Rinsho Ketsueki. 2024. PMID: 39358295 Review. Japanese.
Cited by
-
Deletion of Mecom in mouse results in early-onset spinal deformity and osteopenia.Bone. 2014 Mar;60:148-61. doi: 10.1016/j.bone.2013.11.020. Epub 2013 Dec 4. Bone. 2014. PMID: 24316420 Free PMC article.
-
Evi1 defines leukemia-initiating capacity and tyrosine kinase inhibitor resistance in chronic myeloid leukemia.Oncogene. 2014 Oct 16;33(42):5028-38. doi: 10.1038/onc.2014.108. Epub 2014 Apr 21. Oncogene. 2014. PMID: 24747972 Free PMC article.
-
The oncogene EVI1 enhances transcriptional and biological responses of human myeloid cells to all-trans retinoic acid.Cell Cycle. 2014;13(18):2931-43. doi: 10.4161/15384101.2014.946869. Cell Cycle. 2014. PMID: 25486480 Free PMC article.
-
Common features of megakaryocytes and hematopoietic stem cells: what's the connection?J Cell Biochem. 2009 Aug 1;107(5):857-64. doi: 10.1002/jcb.22184. J Cell Biochem. 2009. PMID: 19492306 Free PMC article. Review.
-
Mice carrying a hypomorphic Evi1 allele are embryonic viable but exhibit severe congenital heart defects.PLoS One. 2014 Feb 27;9(2):e89397. doi: 10.1371/journal.pone.0089397. eCollection 2014. PLoS One. 2014. PMID: 24586749 Free PMC article.
References
-
- Hoyt PR, Bartholomew C, Davis AJ, Yutzey K, Gamer LW, Potter SS, Ihle JN, Mucenski ML (1997) The Evi1 proto-oncogene is required at midgestation for neural, heart, and paraxial mesenchyme development. Mech Dev 65: 55–70 - PubMed
-
- Iscove NN, Sieber F, Winterhalter KH (1974) Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin. J Cell Physiol 83: 309–320 - PubMed
-
- Kaneko S, Onodera M, Fujiki Y, Nagasawa T, Nakauchi H (2001) Simplified retroviral vector GCsap with murine stem cell virus long terminal repeat allows high and continued expression of enhanced green fluorescent protein by human hematopoietic progenitors engrafted in nonobese diabetic/severe combined immunodeficient mice. Hum Gene Ther 12: 35–44 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

