Formation of a novel surface structure encoded by Salmonella Pathogenicity Island 2
- PMID: 15889142
- PMCID: PMC1142609
- DOI: 10.1038/sj.emboj.7600676
Formation of a novel surface structure encoded by Salmonella Pathogenicity Island 2
Abstract
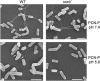
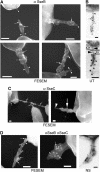
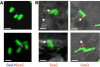
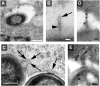
The type III secretion system (T3SS) encoded by Salmonella Pathogenicity Island 2 (SPI2) is essential for virulence and intracellular proliferation of Salmonella enterica. We have previously identified SPI2-encoded proteins that are secreted and function as a translocon for the injection of effector proteins. Here, we describe the formation of a novel SPI2-dependent appendage structure in vitro as well as on the surface of bacteria that reside inside a vacuole of infected host cells. In contrast to the T3SS of other pathogens, the translocon encoded by SPI2 is only present singly or in few copies at one pole of the bacterial cell. Under in vitro conditions, appendages are composed of a filamentous needle-like structure with a diameter of 10 nm that was sheathed with secreted protein. The formation of the appendage in vitro is dependent on acidic media conditions. We analyzed SPI2-encoded appendages in infected cells and observed that acidic vacuolar pH was not required for induction of SPI2 gene expression, but was essential for the assembly of these structures and their function as translocon for delivery of effector proteins.
Figures


Similar articles
-
SseL is a salmonella-specific translocated effector integrated into the SsrB-controlled salmonella pathogenicity island 2 type III secretion system.Infect Immun. 2007 Feb;75(2):574-80. doi: 10.1128/IAI.00985-06. Epub 2006 Dec 11. Infect Immun. 2007. PMID: 17158898 Free PMC article.
-
Regulation of Salmonella pathogenicity island 2 genes by independent environmental signals.Int J Med Microbiol. 2006 Nov;296(7):435-47. doi: 10.1016/j.ijmm.2006.05.001. Epub 2006 Aug 14. Int J Med Microbiol. 2006. PMID: 16904940
-
Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner.Traffic. 2006 Jun;7(6):716-30. doi: 10.1111/j.1600-0854.2006.00422.x. Epub 2006 Apr 21. Traffic. 2006. PMID: 16637890
-
Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells.Cell Microbiol. 2006 May;8(5):728-37. doi: 10.1111/j.1462-5822.2006.00706.x. Cell Microbiol. 2006. PMID: 16611223 Review.
-
Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium.Curr Opin Microbiol. 2007 Feb;10(1):24-9. doi: 10.1016/j.mib.2006.12.002. Epub 2007 Jan 5. Curr Opin Microbiol. 2007. PMID: 17208038 Review.
Cited by
-
σS-Mediated Stress Response Induced by Outer Membrane Perturbation Dampens Virulence in Salmonella enterica serovar Typhimurium.Front Microbiol. 2021 Sep 30;12:750940. doi: 10.3389/fmicb.2021.750940. eCollection 2021. Front Microbiol. 2021. PMID: 34659184 Free PMC article.
-
Control of Salmonella pathogenicity island-2 gene expression.Curr Opin Microbiol. 2009 Apr;12(2):199-204. doi: 10.1016/j.mib.2009.01.004. Epub 2009 Mar 4. Curr Opin Microbiol. 2009. PMID: 19264535 Free PMC article. Review.
-
Salmonella pathogenicity island 2 is expressed prior to penetrating the intestine.PLoS Pathog. 2005 Nov;1(3):e32. doi: 10.1371/journal.ppat.0010032. Epub 2005 Nov 18. PLoS Pathog. 2005. PMID: 16304611 Free PMC article.
-
Localization of EccA3 at the growing pole in Mycobacterium smegmatis.BMC Microbiol. 2022 May 19;22(1):140. doi: 10.1186/s12866-022-02554-6. BMC Microbiol. 2022. PMID: 35590245 Free PMC article.
-
SsaV Interacts with SsaL to Control the Translocon-to-Effector Switch in the Salmonella SPI-2 Type Three Secretion System.mBio. 2018 Oct 2;9(5):e01149-18. doi: 10.1128/mBio.01149-18. mBio. 2018. PMID: 30279280 Free PMC article.
References
-
- Beuzon CR, Banks G, Deiwick J, Hensel M, Holden DW (1999) pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol Microbiol 33: 806–816 - PubMed
-
- Cirillo DM, Valdivia RH, Monack DM, Falkow S (1998) Macrophage-dependent induction of the Salmonella Pathogenicity Island 2 type III secretion system and its role in intracellular survival. Mol Microbiol 30: 175–188 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
