Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli
- PMID: 15889143
- PMCID: PMC1142608
- DOI: 10.1038/sj.emboj.7600674
Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli
Abstract
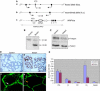
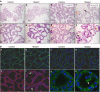
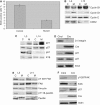
Integrin-extracellular matrix interactions play important roles in the coordinated integration of external and internal cues that are essential for proper development. To study the role of beta1 integrin in the mammary gland, Itgbeta1(flox/flox) mice were crossed with WAPiCre transgenic mice, which led to specific ablation of beta1 integrin in luminal alveolar epithelial cells. In the beta1 integrin mutant mammary gland, individual alveoli were disorganized resulting from alterations in cell-basement membrane associations. Activity of focal adhesion kinase (FAK) was also decreased in mutant mammary glands. Luminal cell proliferation was strongly inhibited in beta1 integrin mutant glands, which correlated with a specific increase of p21 Cip1 expression. In a p21 Cip1 null background, there was a partial rescue of BrdU incorporation, providing in vivo evidence linking p21 Cip1 to the proliferative defect observed in beta1 integrin mutant glands. A connection between p21 Cip1 and beta1 integrin as well as FAK was also established in primary mammary cells. These results point to the essential role of beta1 integrin signaling in mammary epithelial cell proliferation.
Figures




Similar articles
-
Perturbation of beta1-integrin function alters the development of murine mammary gland.EMBO J. 1998 Apr 15;17(8):2139-47. doi: 10.1093/emboj/17.8.2139. EMBO J. 1998. PMID: 9545227 Free PMC article.
-
Decreased expression of beta1-integrin and focal adhesion kinase in epithelial cells may initiate involution of mammary glands.J Cell Physiol. 2004 Aug;200(2):318-25. doi: 10.1002/jcp.20011. J Cell Physiol. 2004. PMID: 15174102
-
Apoptosis and involution in the mammary gland are altered in mice lacking a novel receptor, beta1,4-Galactosyltransferase I.Dev Biol. 2004 Aug 15;272(2):286-309. doi: 10.1016/j.ydbio.2004.03.041. Dev Biol. 2004. PMID: 15282149
-
[Mammary gland development: Role of basal myoepithelial cells].J Soc Biol. 2006;200(2):193-8. doi: 10.1051/jbio:2006021. J Soc Biol. 2006. PMID: 17151555 Review. French.
-
Integrin signaling and mammary cell function.J Mammary Gland Biol Neoplasia. 2003 Oct;8(4):395-408. doi: 10.1023/B:JOMG.0000017427.14751.8c. J Mammary Gland Biol Neoplasia. 2003. PMID: 14985636 Review.
Cited by
-
How integrins control breast biology.Curr Opin Cell Biol. 2013 Oct;25(5):633-41. doi: 10.1016/j.ceb.2013.06.010. Epub 2013 Jul 22. Curr Opin Cell Biol. 2013. PMID: 23886475 Free PMC article. Review.
-
β1 integrin deletion enhances progression of prostate cancer in the TRAMP mouse model.Sci Rep. 2012;2:526. doi: 10.1038/srep00526. Epub 2012 Jul 24. Sci Rep. 2012. PMID: 22829980 Free PMC article.
-
Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner.EMBO J. 2007 Mar 21;26(6):1487-98. doi: 10.1038/sj.emboj.7601599. Epub 2007 Feb 22. EMBO J. 2007. PMID: 17318179 Free PMC article.
-
The Role of Extracellular Matrix Proteins in Breast Cancer.J Clin Med. 2022 Feb 25;11(5):1250. doi: 10.3390/jcm11051250. J Clin Med. 2022. PMID: 35268340 Free PMC article. Review.
-
Beta1-integrin is required for kidney collecting duct morphogenesis and maintenance of renal function.Am J Physiol Renal Physiol. 2009 Jul;297(1):F210-7. doi: 10.1152/ajprenal.90260.2008. Epub 2009 May 13. Am J Physiol Renal Physiol. 2009. PMID: 19439520 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

