The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth
- PMID: 15889145
- PMCID: PMC1142606
- DOI: 10.1038/sj.emboj.7600672
The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth
Abstract
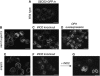
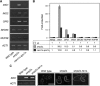
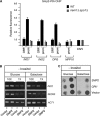
Remodelling of the nuclear membrane is essential for the dynamic changes of nuclear architecture at different stages of the cell cycle and during cell differentiation. The molecular mechanism underlying the regulation of nuclear membrane biogenesis is not known. Here we show that Smp2, the yeast homologue of mammalian lipin, is a key regulator of nuclear membrane growth during the cell cycle. Smp2 is phosphorylated by Cdc28/Cdk1 and dephosphorylated by a nuclear/endoplasmic reticulum (ER) membrane-localized CPD phosphatase complex consisting of Nem1 and Spo7. Loss of either SMP2 or its dephosphorylated form causes transcriptional upregulation of key enzymes involved in lipid biosynthesis concurrent with a massive expansion of the nucleus. Conversely, constitutive dephosphorylation of Smp2 inhibits cell division. We show that Smp2 associates with the promoters of phospholipid biosynthetic enzymes in a Nem1-Spo7-dependent manner. Our data suggest that Smp2 is a critical factor in coordinating phospholipid biosynthesis at the nuclear/ER membrane with nuclear growth during the cell cycle.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

