Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion
- PMID: 15889152
- PMCID: PMC1142591
- DOI: 10.1038/sj.emboj.7600658
Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion
Abstract
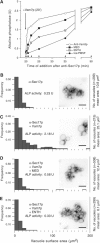
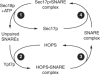
SNARE functions during membrane docking and fusion are regulated by Sec1/Munc18 (SM) chaperones and Rab/Ypt GTPase effectors. These functions for yeast vacuole fusion are combined in the six-subunit HOPS complex. HOPS facilitates Ypt7p nucleotide exchange, is a Ypt7p effector, and contains an SM protein. We have dissected the associations and requirements for HOPS, Ypt7p, and Sec17/18p during SNARE complex assembly. Vacuole SNARE complexes bind either Sec17p or the HOPS complex, but not both. Sec17p and its co-chaperone Sec18p disassemble SNARE complexes. Ypt7p regulates the reassembly of unpaired SNAREs with each other and with HOPS, forming HOPS.SNARE complexes prior to fusion. After HOPS.SNARE assembly, lipid rearrangements are still required for vacuole content mixing. Thus, Sec17p and HOPS have mutually exclusive interactions with vacuole SNAREs to mediate disruption of SNARE complexes or their assembly for docking and fusion. Sec17p may displace HOPS from SNAREs to permit subsequent rounds of fusion.
Figures





References
-
- Allan BB, Moyer BD, Balch WE (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289: 444–448 - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current Protocols in Molecular Biology. New York: John Wiley & Sons Inc.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

