Impaired maturation of myeloid progenitors in mice lacking novel Polycomb group protein MBT-1
- PMID: 15889154
- PMCID: PMC1142590
- DOI: 10.1038/sj.emboj.7600654
Impaired maturation of myeloid progenitors in mice lacking novel Polycomb group protein MBT-1
Abstract
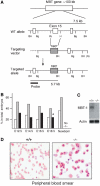
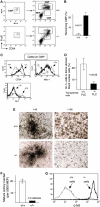
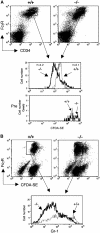
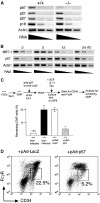
Polycomb group (PcG) proteins participate in DNA-binding complexes with gene-repressing activity, many of which have been highlighted for their involvement in hematopoiesis. We have identified a putative PcG protein, termed MBT-1, that is associated with Rnf2, an in vivo interactor of PcG proteins. MBT-1 structurally resembles the H-L(3)MBT protein, whose deletion is predicted to be responsible for myeloid hematopoietic malignancies. The human MBT-1 gene is located on chromosome 6q23, a region frequently deleted in leukemia cells, and shows a transient expression spike in response to maturation-inducing stimuli in myeloid leukemia cells. MBT-1(-/-) myeloid progenitor cells exhibit a maturational deficiency but maintain normal proliferative activities. This results in the accumulation of immature myeloid progenitors and hence, a marked decrease of mature myeloid blood cells, causing the MBT-1(-/-) mice to die of anemia during a late embryonic stage. Together, we conclude that MBT-1 specifically regulates the maturational advancement of myeloid progenitor cells during transitions between two developmental stages. We also show that MBT-1 appears to influence myelopoiesis by transiently enhancing p57(KIP2) expression levels.
Figures



Similar articles
-
The human L(3)MBT polycomb group protein is a transcriptional repressor and interacts physically and functionally with TEL (ETV6).J Biol Chem. 2003 Apr 25;278(17):15412-20. doi: 10.1074/jbc.M300592200. Epub 2003 Feb 14. J Biol Chem. 2003. PMID: 12588862
-
Reciprocal expression of Bmi1 and Mel-18 is associated with functioning of primitive hematopoietic cells.Exp Hematol. 2009 Jul;37(7):857-866.e2. doi: 10.1016/j.exphem.2009.04.011. Epub 2009 May 3. Exp Hematol. 2009. PMID: 19409954
-
The polycomb group gene product Ezh2 regulates proliferation and differentiation of murine hepatic stem/progenitor cells.J Hepatol. 2010 Jun;52(6):854-63. doi: 10.1016/j.jhep.2010.01.027. Epub 2010 Mar 24. J Hepatol. 2010. PMID: 20395008
-
Molecular basis for stem-cell self-renewal.Hematol J. 2004;5 Suppl 3:S118-21. doi: 10.1038/sj.thj.6200436. Hematol J. 2004. PMID: 15190292 Review. No abstract available.
-
[Regulatory effects of Bmi-1 gene on self-renewal of hematopoietic stem cells--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006 Apr;14(2):413-5. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006. PMID: 16638228 Review. Chinese.
Cited by
-
L3MBTL3 and STAT3 collaboratively upregulate SNAIL expression to promote metastasis in female breast cancer.Nat Commun. 2025 Jan 2;16(1):231. doi: 10.1038/s41467-024-55617-9. Nat Commun. 2025. PMID: 39747894 Free PMC article.
-
Chromatin protein L3MBTL1 is dispensable for development and tumor suppression in mice.J Biol Chem. 2010 Sep 3;285(36):27767-75. doi: 10.1074/jbc.M110.115410. Epub 2010 Jun 30. J Biol Chem. 2010. PMID: 20592034 Free PMC article.
-
Small-molecule ligands of methyl-lysine binding proteins: optimization of selectivity for L3MBTL3.J Med Chem. 2013 Sep 26;56(18):7358-71. doi: 10.1021/jm400919p. Epub 2013 Sep 16. J Med Chem. 2013. PMID: 24040942 Free PMC article.
-
Methylated DNMT1 and E2F1 are targeted for proteolysis by L3MBTL3 and CRL4DCAF5 ubiquitin ligase.Nat Commun. 2018 Apr 24;9(1):1641. doi: 10.1038/s41467-018-04019-9. Nat Commun. 2018. PMID: 29691401 Free PMC article.
-
MBTD1 preserves adult hematopoietic stem cell pool size and function.Proc Natl Acad Sci U S A. 2023 Aug 8;120(32):e2206860120. doi: 10.1073/pnas.2206860120. Epub 2023 Jul 31. Proc Natl Acad Sci U S A. 2023. PMID: 37523546 Free PMC article.
References
-
- Akasaka T, Tsuji K, Kawahira H, Kanno M, Harigaya K, Hu L, Ebihara Y, Nakahata T, Tetsu O, Taniguchi M, Koseki H (1997) The role of mel-18, a mammalian Polycomb group gene, during IL-7-dependent proliferation of lymphocyte precursors. Immunity 7: 135–146 - PubMed
-
- Akashi K, Reya T, Dalma-Weiszhausz D, Weissman IL (2000a) Lymphoid precursors. Curr Opin Immunol 12: 144–150 - PubMed
-
- Akashi K, Traver D, Miyamoto T, Weissman IL (2000b) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197 - PubMed
-
- Alkema MJ, Jacobs H, van Lohuizen M, Berns A (1997) Pertubation of B and T cell development and predisposition to lymphomagenesis in Emu Bmi1 transgenic mice require the Bmi1 RING finger. Oncogene 15: 899–910 - PubMed
-
- Barreda DR, Belosevic M (2001) Transcriptional regulation of hemopoiesis. Dev Comp Immunol 25: 763–789 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

