Anti-tumor immune responses following neoadjuvant immunotherapy with a recombinant adenovirus expressing HSP72 to rodent tumors
- PMID: 15889253
- PMCID: PMC11034332
- DOI: 10.1007/s00262-005-0683-4
Anti-tumor immune responses following neoadjuvant immunotherapy with a recombinant adenovirus expressing HSP72 to rodent tumors
Abstract
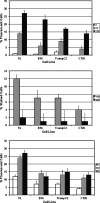
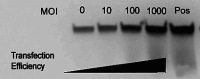
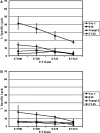
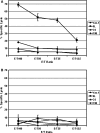
Gene modification of tumor cells is commonly utilized in various strategies of immunotherapy preventive both as treatment and a means to modify tumor growth. Gene transfer prior to surgery as neoadjuvant therapy has not been studied systematically. We addressed, whether direct intra-tumoral injection of a recombinant adenovirus expressing the immunomodulatory molecule, heat shock protein 72 (ADHSP72), administered prior to surgery could result in sustainable anti-tumor immune responses capable of affecting tumor progression and survival in a number of different murine and rat tumor models. Using intra-dermal murine models of melanoma (B16), colorectal carcinoma (CT26), prostate cancer (TrampC2) and a rat model of glioblastoma (9L), tumors were treated with vehicle or GFP expressing adenovirus (ADGFP) or ADHSP72. Tumors were surgically excised after 72 h. Approximately 25-50% of animals in the ADHSP72 treatment group but not in control groups showed sustained resistance to subsequent tumor challenge. Tumor resistance was associated with development of anti-tumor cellular immune responses. Efficacy of ADHSP72 as neoadjuvant therapy was dependent on the size of the initial tumor with greater likelihood of immune response generation and tumor resistance associated with smaller tumor size at initial treatment. ADHSP72 neoadjuvant therapy resulted in prolonged survival of animals upon re-challenge with autologous tumor cells compared to ADGFP or vehicle control groups. To study the effects on tumor progression of distant metastases, a single tumor focus of animals with multifocal intra-dermal tumors was treated. ADHSP72 diminished progression of the secondary tumor focus and prolonged survival, but only when the secondary tumor focus was <50 mm3 . Our results indicate that gene modification of tumors prior to surgical intervention may be beneficial to prevent recurrence in specific circumstances.
Figures




Similar articles
-
Enhanced antitumoral efficacy and immune response following conditionally replicative adenovirus containing constitutive HSF1 delivery to rodent tumors.J Transl Med. 2012 May 21;10:101. doi: 10.1186/1479-5876-10-101. J Transl Med. 2012. PMID: 22613625 Free PMC article.
-
Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts.Cancer Res. 2004 May 1;64(9):3281-7. doi: 10.1158/0008-5472.can-03-3911. Cancer Res. 2004. PMID: 15126371
-
Tumor immunity and prolonged survival following combined adenovirus-HSP72 and CEA-plasmid vaccination.Vaccine. 2005 May 20;23(27):3565-71. doi: 10.1016/j.vaccine.2005.01.148. Vaccine. 2005. PMID: 15855015
-
Anti-tumor immunity induced by in vivo adenovirus vector-mediated expression of CD40 ligand in tumor cells.Hum Gene Ther. 1999 May 20;10(8):1375-87. doi: 10.1089/10430349950018049. Hum Gene Ther. 1999. PMID: 10365667
-
Cancer, aging and immunotherapy: lessons learned from animal models.Cancer Immunol Immunother. 2009 Dec;58(12):1979-89. doi: 10.1007/s00262-009-0677-8. Epub 2009 Feb 24. Cancer Immunol Immunother. 2009. PMID: 19238382 Free PMC article. Review.
Cited by
-
Enhanced antitumoral efficacy and immune response following conditionally replicative adenovirus containing constitutive HSF1 delivery to rodent tumors.J Transl Med. 2012 May 21;10:101. doi: 10.1186/1479-5876-10-101. J Transl Med. 2012. PMID: 22613625 Free PMC article.
-
Characterization of surgical models of postoperative tumor recurrence for preclinical adjuvant therapy assessment.Am J Transl Res. 2012;4(2):206-18. Epub 2012 Apr 10. Am J Transl Res. 2012. PMID: 22611473 Free PMC article.
-
Expression of chemoresistance-related genes and heat shock protein 72 in hyperthermic isolated limb perfusion of malignant melanoma: an experimental study.J Oncol. 2010;2010:138758. doi: 10.1155/2010/138758. Epub 2010 Jun 20. J Oncol. 2010. PMID: 20634932 Free PMC article.
References
-
- Quan WD, Jr, Palackdharry CS. Common cancers–immunotherapy and multidisciplinary therapy: parts III and IV. Dis Mon. 1997;43:745–808. - PubMed
-
- Buter J, Pinedo HM. Neoadjuvant chemoimmunotherapy in locally advanced breast cancer: a new avenue to be explored. Curr Oncol Rep. 2003;5:171–176. - PubMed
-
- Bodar E, et al. Immunologic and biologic properties of the primary tumor during prolonged neoadjuvant chemoimmunotherapy. Oncology (Huntingt) 2002;16:32–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

