Inhibition of APC anticoagulant activity on oxidized phospholipid by anti-{beta}2-glycoprotein I monoclonal antibodies
- PMID: 15890686
- PMCID: PMC1895229
- DOI: 10.1182/blood-2005-01-0404
Inhibition of APC anticoagulant activity on oxidized phospholipid by anti-{beta}2-glycoprotein I monoclonal antibodies
Abstract
Activated protein C (APC) anticoagulant activity and the ability to be inhibited by auto-antibodies associated with thrombosis are strongly augmented by the presence of phosphatidylethanolamine (PE) and phospholipid oxidation. beta(2)-glycoprotein I (beta(2)-GPI) is a major antigen for antiphospholipid antibodies present in patients with the antiphospholipid syndrome. We therefore investigated whether anti-beta(2)-GPI monoclonal antibodies (mAbs) could inhibit APC with similar membrane specificity. Five mouse mAbs that reacted with different epitopes on beta(2)-GPI were examined. Each inhibited the PE-, phospholipid oxidation-dependent enhancement of APC anticoagulant activity and required antibody divalency. A chimeric APC that retains anticoagulant activity but is relatively unaffected by protein S, PE, or oxidation was not inhibited by the antibodies. In purified systems, anti-beta(2)-GPI mAb inhibition of factor Va inactivation was greater in the presence of protein S and required beta(2)-GPI. Surprisingly, although the mAbs did increase beta(2)-GPI affinity for membranes, PE and oxidation had little influence on the affinity of the beta(2)-GPI antibody complex for the membrane vesicles. We conclude that antibodies to beta(2)-GPI inhibit APC function specifically and contribute to a hypercoaguable state by disrupting specific protein-protein interactions induced by oxidation of PE-containing membranes.
Figures

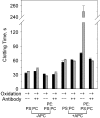
 ) in the presence or absence of anti-β2 mAb no. 1522 (120 nM) and APC (0.4 μg/mL) as indicated on the x-axis. Similar results were obtained with the other antibodies. Error bars indicate the SE of 2 or 3 determinations run in duplicate.
) in the presence or absence of anti-β2 mAb no. 1522 (120 nM) and APC (0.4 μg/mL) as indicated on the x-axis. Similar results were obtained with the other antibodies. Error bars indicate the SE of 2 or 3 determinations run in duplicate.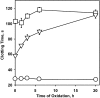

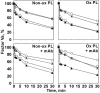
 ) and used in clotting assays in the presence or absence of 250 nM anti–β2-GPI mAb no. 1522 and anticoagulant enzyme as indicated on the x-axis. Similar results were obtained with APC and APC-PtGla with the other antibodies. Error bars indicate the SE of 2 determinations run in duplicate.
) and used in clotting assays in the presence or absence of 250 nM anti–β2-GPI mAb no. 1522 and anticoagulant enzyme as indicated on the x-axis. Similar results were obtained with APC and APC-PtGla with the other antibodies. Error bars indicate the SE of 2 determinations run in duplicate.

References
-
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346: 752-763. - PubMed
-
- Inanc M, Radway-Bright EL, Isenberg DA. β2-Glycoprotein I and anti-β2-glycoprotein I antibodies: where are we now? Br J Rheumatol. 1997; 36: 1247-1257. - PubMed
-
- Safa O, Hensley K, Smirnov MD, Esmon CT, Esmon NL. Lipid oxidation enhances the function of activated protein C. J Biol Chem. 2001;276: 1829-1836. - PubMed
-
- Marciniak E, Romond EH. Impaired catalytic function of activated protein C: a new in vitro manifestation of lupus anticoagulant. Blood. 1989;74: 2426-2432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

