Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy
- PMID: 15890688
- PMCID: PMC1895219
- DOI: 10.1182/blood-2004-11-4365
Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy
Abstract
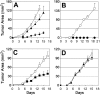
NKG2D is an activating cell-surface receptor expressed on natural killer (NK) cells and some T-cell subsets. Its ligands are primarily expressed on tumor cells. The aim of this study was to determine whether chimeric NK-receptor-bearing T cells would directly kill tumor cells and lead to induction of host immunity against tumors. Chimeric NK receptors were produced by linking NKG2D or DNAX activating protein of 10 kDa (Dap10) to the cytoplasmic portion of the CD3zeta chain. Our results showed that chimeric (ch) NKG2D-bearing T cells responded to NKG2D-ligand-bearing tumor cells (RMA/Rae-1beta, EG7) but not to wild-type tumor cells (RMA). This response was dependent upon ligand expression on the target cells but not on expression of major histocompatibility complex (MHC) molecules, and the response could be blocked by anti-NKG2D antibodies. These T cells produced large amounts of T-helper 1 (Th1) cytokines and proinflammatory chemokines and killed ligand-expressing tumor cells. Adoptive transfer of chNKG2D-bearing T cells inhibited RMA/Rae-1beta tumor growth in vivo. Moreover, mice that had remained tumor-free were resistant to subsequent challenge with the wild-type RMA tumor cells, suggesting the generation of immunity against other tumor antigens. Taken together, our findings indicate that modification of T cells with chimeric NKG2D receptors represents a promising approach for immunotherapy against cancer.
Figures


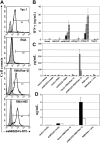
 ), RMA/Rae-1β (▪), RMA/H60 (
), RMA/Rae-1β (▪), RMA/H60 ( ), and YAC-1 (
), and YAC-1 ( ) or media alone (□) for 24 hours. Concentrations of IFN-γ in supernatants were determined by ELISA. For detection of other cytokines (C) and chemokines (D), irradiated (100 Gy) tumor cells instead were mixed with T cells for 3 days. Bio-plex assays were performed to measure the levels of GM-CSF (
) or media alone (□) for 24 hours. Concentrations of IFN-γ in supernatants were determined by ELISA. For detection of other cytokines (C) and chemokines (D), irradiated (100 Gy) tumor cells instead were mixed with T cells for 3 days. Bio-plex assays were performed to measure the levels of GM-CSF ( ), IL-3 (
), IL-3 ( ), IL-5 (
), IL-5 ( ), IL-10 (▪) (shown in C), CCL3 (▪), and CCL5 (□) (D).
), IL-10 (▪) (shown in C), CCL3 (▪), and CCL5 (□) (D).

References
-
- Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10: 5-18. - PubMed
-
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3: 35-45. - PubMed
-
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411: 380-384. - PubMed
-
- Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3: 431-437. - PubMed
-
- Crowley NJ, Slingluff CL Jr, Darrow TL, Seigler HF. Generation of human autologous melanoma-specific cytotoxic T-cells using HLA-A2-matched allogeneic melanomas. Cancer Res. 1990;50: 492-498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

