Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells
- PMID: 15890705
- PMCID: PMC1464792
- DOI: 10.1113/jphysiol.2005.086835
Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells
Abstract
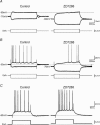
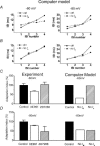
In hippocampal pyramidal cells, a single action potential (AP) or a burst of APs is followed by a medium afterhyperpolarization (mAHP, lasting approximately 0.1 s). The currents underlying the mAHP are considered to regulate excitability and cause early spike frequency adaptation, thus dampening the response to sustained excitatory input relative to responses to abrupt excitation. The mAHP was originally suggested to be primarily caused by M-channels (at depolarized potentials) and h-channels (at more negative potentials), but not SK channels. In recent reports, however, the mAHP was suggested to be generated mainly by SK channels or only by h-channels. We have now re-examined the mechanisms underlying the mAHP and early spike frequency adaptation in CA1 pyramidal cells by using sharp electrode and whole-cell recording in rat hippocampal slices. The specific M-channel blocker XE991 (10 microm) suppressed the mAHP following 1-5 APs evoked by current injection at -60 mV. XE991 also enhanced the excitability of the cell, i.e. increased the number of APs evoked by a constant depolarizing current pulse, reduced their rate of adaptation, enhanced the after depolarization and promoted bursting. Conversely, the M-channel opener retigabine reduced excitability. The h-channel blocker ZD7288 (4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride; 10 microm) fully suppressed the mAHP at -80 mV, but had little effect at -60 mV, whereas XE991 did not measurably affect the mAHP at -80 mV. Likewise, ZD7288 had little or no effect on excitability or adaptation during current pulses injected from -60 mV, but changed the initial discharge during depolarizing pulses injected from -80 mV. In contrast to previous reports, we found that blockade of Ca2+-activated K+ channels of the SK/KCa type by apamin (100-400 nm) failed to affect the mAHP or adaptation. A computational model of a CA1 pyramidal cell predicted that M- and h-channels will generate mAHPs in a voltage-dependent manner, as indicated by the experiments. We conclude that M- and h-channels generate the somatic mAHP in hippocampal pyramidal cells, with little or no net contribution from SK channels.
Figures











Similar articles
-
SK (KCa2) channels do not control somatic excitability in CA1 pyramidal neurons but can be activated by dendritic excitatory synapses and regulate their impact.J Neurophysiol. 2008 Nov;100(5):2589-604. doi: 10.1152/jn.90433.2008. Epub 2008 Aug 6. J Neurophysiol. 2008. PMID: 18684909
-
Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells.J Neurophysiol. 2006 Jun;95(6):3480-95. doi: 10.1152/jn.01333.2005. Epub 2006 Feb 22. J Neurophysiol. 2006. PMID: 16495357
-
Small-conductance Ca2+-dependent K+ channels are the target of spike-induced Ca2+ release in a feedback regulation of pyramidal cell excitability.J Neurophysiol. 2004 May;91(5):2322-9. doi: 10.1152/jn.01049.2003. Epub 2003 Dec 24. J Neurophysiol. 2004. PMID: 14695351
-
Ca2+ signalling and Ca2+-activated K+ channels in smooth muscle.Novartis Found Symp. 2002;246:52-64; discussion 64-70, 221-7. Novartis Found Symp. 2002. PMID: 12164315 Review.
-
Potassium currents in hippocampal pyramidal cells.Prog Brain Res. 1990;83:161-87. doi: 10.1016/s0079-6123(08)61248-0. Prog Brain Res. 1990. PMID: 2203097 Review.
Cited by
-
Selective interaction of syntaxin 1A with KCNQ2: possible implications for specific modulation of presynaptic activity.PLoS One. 2009 Aug 13;4(8):e6586. doi: 10.1371/journal.pone.0006586. PLoS One. 2009. PMID: 19675672 Free PMC article.
-
Modulation of SK Channels via Calcium Buffering Tunes Intrinsic Excitability of Parvalbumin Interneurons in Neuropathic Pain: A Computational and Experimental Investigation.J Neurosci. 2023 Aug 2;43(31):5608-5622. doi: 10.1523/JNEUROSCI.0426-23.2023. Epub 2023 Jul 14. J Neurosci. 2023. PMID: 37451982 Free PMC article.
-
Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications.J Physiol. 2008 Apr 1;586(7):1811-21. doi: 10.1113/jphysiol.2007.148304. Epub 2008 Jan 31. J Physiol. 2008. PMID: 18238808 Free PMC article. Review.
-
Acute alterations of somatodendritic action potential dynamics in hippocampal CA1 pyramidal cells after kainate-induced status epilepticus in mice.PLoS One. 2011;6(10):e26664. doi: 10.1371/journal.pone.0026664. Epub 2011 Oct 24. PLoS One. 2011. PMID: 22039527 Free PMC article.
-
IK1 channels do not contribute to the slow afterhyperpolarization in pyramidal neurons.Elife. 2016 Jan 14;5:e11206. doi: 10.7554/eLife.11206. Elife. 2016. PMID: 26765773 Free PMC article.
References
-
- Abel HJ, Lee JC, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol. 2004;91:324–335. - PubMed
-
- Aiken SP, Zaczek R, Brown BS. Pharmacology of the neurotransmitter release enhancer linopirdine (DuP 996), and insights into its mechanism of action. Adv Pharmacol. 1996;35:349–384. - PubMed
-
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. - PubMed
-
- Bond CT, Maylie J, Adelman JP. Small-conductance calcium-activated potassium channels. Ann N Y Acad Sci. 1999;868:370–378. - PubMed
-
- Borgatti R, Zucca C, Cavallini A, Ferrario M, Panzeri C, Castaldo P, Soldovieri MV, Baschirotto C, Bresolin N, Dalla BB, Taglialatela M, Bassi MT. A novel mutation in KCNQ2 associated with BFNC, drug resistant epilepsy, and mental retardation. Neurology. 2004;63:57–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

