The frequencies of calcium oscillations are optimized for efficient calcium-mediated activation of Ras and the ERK/MAPK cascade
- PMID: 15890781
- PMCID: PMC1103707
- DOI: 10.1073/pnas.0409611102
The frequencies of calcium oscillations are optimized for efficient calcium-mediated activation of Ras and the ERK/MAPK cascade
Abstract
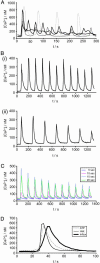
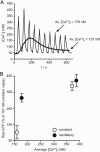
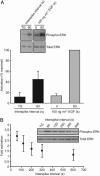
Ras proteins are binary switches that, by cycling through inactive GDP- and active GTP-bound conformations, regulate multiple cellular signaling pathways, including those that control growth and differentiation. For some time, it has been known that receptor-mediated increases in the concentration of intracellular free calcium ([Ca(2+)](i)) can modulate Ras activation. Increases in [Ca(2+)](i) often occur as repetitive Ca(2+) spikes or oscillations. Induced by electrical or receptor stimuli, these repetitive Ca(2+) oscillations increase in frequency with the amplitude of receptor stimuli, a phenomenon critical for the induction of selective cellular functions. Here, we show that Ca(2+) oscillations are optimized for Ca(2+)-mediated activation of Ras and signaling through the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) cascade. We present additional evidence that Ca(2+) oscillations reduce the effective Ca(2+) threshold for the activation of Ras and that the oscillatory frequency is optimized for activation of Ras and the ERK/MAPK pathway. Our results describe a hitherto unrecognized link between complex Ca(2+) signals and the modulation of the Ras/ERK/MAPK signaling cascade.
Figures

Similar articles
-
Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation.J Neurosci. 2005 Feb 2;25(5):1281-90. doi: 10.1523/JNEUROSCI.4086-04.2005. J Neurosci. 2005. PMID: 15689566 Free PMC article.
-
MEK and ERK activation in ras-disabled RBL-2H3 mast cells and novel roles for geranylgeranylated and farnesylated proteins in Fc epsilonRI-mediated signaling.J Immunol. 1998 Dec 15;161(12):6733-44. J Immunol. 1998. PMID: 9862703
-
Constitutively active Galpha16 stimulates STAT3 via a c-Src/JAK- and ERK-dependent mechanism.J Biol Chem. 2003 Dec 26;278(52):52154-65. doi: 10.1074/jbc.M307299200. Epub 2003 Oct 9. J Biol Chem. 2003. PMID: 14551213
-
Control of Ras cycling by Ca2+.FEBS Lett. 2003 Jul 3;546(1):6-10. doi: 10.1016/s0014-5793(03)00412-5. FEBS Lett. 2003. PMID: 12829229 Review.
-
The Ras binary switch: an ideal processor for decoding complex Ca2+ signals?Biochem Soc Trans. 2003 Oct;31(Pt 5):966-9. doi: 10.1042/bst0310966. Biochem Soc Trans. 2003. PMID: 14505461 Review.
Cited by
-
Purinergic Ca2+ signaling as a novel mechanism of drug tolerance in BRAF mutant melanoma.bioRxiv [Preprint]. 2024 Apr 1:2023.11.03.565532. doi: 10.1101/2023.11.03.565532. bioRxiv. 2024. Update in: Cancers (Basel). 2024 Jun 30;16(13):2426. doi: 10.3390/cancers16132426. PMID: 37961267 Free PMC article. Updated. Preprint.
-
Scrutinizing calcium flux oscillations in T lymphocytes to deduce the strength of stimulus.Sci Rep. 2015 Jan 14;5:7760. doi: 10.1038/srep07760. Sci Rep. 2015. PMID: 25585590 Free PMC article.
-
Estrogen signaling multiple pathways to impact gene transcription.Curr Genomics. 2006;7(8):497-508. doi: 10.2174/138920206779315737. Curr Genomics. 2006. PMID: 18369406 Free PMC article.
-
Endothelial mitochondria regulate the intracellular Ca2+ response to fluid shear stress.Am J Physiol Cell Physiol. 2016 Mar 15;310(6):C479-90. doi: 10.1152/ajpcell.00171.2015. Epub 2016 Jan 6. Am J Physiol Cell Physiol. 2016. PMID: 26739489 Free PMC article.
-
Band-pass processing in a GPCR signaling pathway selects for NFAT transcription factor activation.Integr Biol (Camb). 2015 Nov;7(11):1378-86. doi: 10.1039/c5ib00181a. Integr Biol (Camb). 2015. PMID: 26374065 Free PMC article.
References
-
- Berridge, M. J., Lipp, P. & Bootman, M. D. (2000) Nat. Rev. Mol. Cell Biol. 1, 11–21. - PubMed
-
- Bootman, M. D., Lipp, P. & Berridge, M. J. (2001) J. Cell Sci. 114, 2213–2222. - PubMed
-
- Berridge, M. J., Bootman, M. D. & Roderick, H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529. - PubMed
-
- Berridge, M. J. & Prince, W. T. (1972) J. Exp. Biol. 56, 139–153. - PubMed
-
- Woods, N. M., Cuthbertson, K. S. R. & Cobbold, P. H. (1986) Nature 319, 600–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

