Selective replication of coronavirus genomes that express nucleocapsid protein
- PMID: 15890900
- PMCID: PMC1112145
- DOI: 10.1128/JVI.79.11.6620-6630.2005
Selective replication of coronavirus genomes that express nucleocapsid protein
Abstract
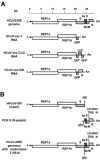
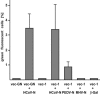
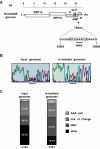
The coronavirus nucleocapsid (N) protein is a structural protein that forms a ribonucleoprotein complex with genomic RNA. In addition to its structural role, it has been described as an RNA-binding protein that might be involved in coronavirus RNA synthesis. Here, we report a reverse genetic approach to elucidate the role of N in coronavirus replication and transcription. We found that human coronavirus 229E (HCoV-229E) vector RNAs that lack the N gene were greatly impaired in their ability to replicate, whereas the transcription of subgenomic mRNA from these vectors was easily detectable. In contrast, vector RNAs encoding a functional N protein were able to carry out both replication and transcription. Furthermore, modification of the transcription signal required for the synthesis of N protein mRNAs in the HCoV-229E genome resulted in the selective replication of genomes that are able to express the N protein. This genetic evidence leads us to conclude that at least one coronavirus structural protein, the N protein, is involved in coronavirus replication.
Figures



References
-
- Barr, J. N., S. P. Whelan, and G. W. Wertz. 2002. Transcriptional control of the RNA-dependent RNA polymerase of vesicular stomatitis virus. Biochim. Biophys. Acta 1577:337-353. - PubMed
-
- Blumberg, B. M., M. Leppert, and D. Kolakofsky. 1981. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell 23:837-845. - PubMed
-
- Bol, J. F. 1999. Alfalfa mosaic virus and ilarviruses: involvement of coat protein in multiple steps of the replication cycle. J. Gen. Virol. 80:1089-1102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

