Interaction of tSNARE syntaxin 18 with the papillomavirus minor capsid protein mediates infection
- PMID: 15890910
- PMCID: PMC1112158
- DOI: 10.1128/JVI.79.11.6723-6731.2005
Interaction of tSNARE syntaxin 18 with the papillomavirus minor capsid protein mediates infection
Abstract
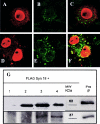
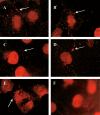
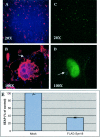
The papillomavirus capsid mediates binding to the cell surface and passage of the virion to the perinuclear region during infection. To better understand how the virus traffics across the cell, we sought to identify cellular proteins that bind to the minor capsid protein L2. We have identified syntaxin 18 as a protein that interacts with bovine papillomavirus type 1 (BPV1) L2. Syntaxin 18 is a target membrane-associated soluble N-ethylmaleimide-sensitive factor-attachment protein receptor (tSNARE) that resides in the endoplasmic reticulum (ER). The ectopic expression of FLAG-tagged syntaxin 18, which disrupts ER trafficking, blocked BPV1 pseudovirion infection. Furthermore, the expression of FLAG-syntaxin 18 prevented the passage of BPV1 pseudovirions to the perinuclear region that is consistent with the ER. Genetic studies identified a highly conserved L2 domain, DKILK, comprising residues 40 to 44 that mediated BPV1 trafficking through the ER during infection via an interaction with the tSNARE syntaxin 18. Mutations within the DKILK motif of L2 that did not significantly impact virion morphogenesis or binding at the cell surface prevented the L2 interaction with syntaxin 18 and disrupted BPV1 infection.
Figures




References
-
- Becker, K. A., L. Florin, C. Sapp, and M. Sapp. 2003. Dissection of human papillomavirus type 33 L2 domains involved in nuclear domains (ND) 10 homing and reorganization. Virology 314:161-167. - PubMed
-
- Booy, F. P., R. B. Roden, H. L. Greenstone, J. T. Schiller, and B. L. Trus. 1998. Two antibodies that neutralize papillomavirus by different mechanisms show distinct binding patterns at 13 A resolution. J. Mol. Biol. 281:95-106. - PubMed
-
- Brobst, D. F., and G. C. Dulac. 1969. Meningeal tumors induced in calves with the bovine cutaneous papilloma virus. Pathol. Vet. 6:135-145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

