The carboxyl-terminal domain of RNA polymerase II is phosphorylated by a complex containing cdk9 and infected-cell protein 22 of herpes simplex virus 1
- PMID: 15890914
- PMCID: PMC1112163
- DOI: 10.1128/JVI.79.11.6757-6762.2005
The carboxyl-terminal domain of RNA polymerase II is phosphorylated by a complex containing cdk9 and infected-cell protein 22 of herpes simplex virus 1
Abstract
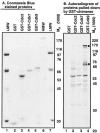
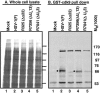
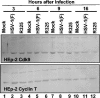
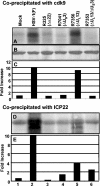
The infected-cell protein 22 (ICP22), a regulatory protein encoded by the alpha22 gene of herpes simplex virus 1, is required for the optimal expression of a set of late viral proteins that includes the products of the U(S)11, U(L)38, and U(L)41 genes. ICP22 has two activities. Thus, ICP22 and the U(L)13 protein kinase mediate the activation of cdc2 and degradation of its partners, cyclins A and B. cdc2 and its new partner, the DNA polymerase accessory factor (U(L)42), bind topoisomerase IIalpha in an ICP22-dependent manner. In addition, ICP22 and U(L)13 mediate an intermediate phosphorylation of the carboxyl terminus of RNA polymerase II (RNA POL II). Here we report another function of ICP22. Thus, ICP22 physically interacts with cdk9, a constitutively active cyclin-dependent kinase involved in transcriptional regulation. A protein complex containing ICP22 and cdk9 phosphorylates in vitro the carboxyl-terminal domain of RNA POL II in a viral U(S)3 protein kinase-dependent fashion. Finally, the carboxyl-terminal domain of RNA POL II fused to glutathione S-transferase is phosphorylated in reaction mixtures containing complexes pulled down with ICP22 or cdk9 immune precipitated from lysates of wild-type parent virus or deltaU(L)13 but not deltaU(S)3 mutant-infected cells. The experiments described here place ICP22 and cdk9 in a complex with the carboxyl-terminal domain of RNA POL II. At the same time we confirm the requirement of ICP22 and the U(L)13 protein kinase in the posttranslational modification of RNA POL II that alters its electrophoretic mobility, although U(S)3 kinase appears to play a role in a cell-type-dependent fashion.
Figures


References
-
- Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in activity of the G2/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the α22/US1.5 and UL13 viral genes. J. Virol. 74:8-15. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

