Poliovirus proteins induce membrane association of GTPase ADP-ribosylation factor
- PMID: 15890959
- PMCID: PMC1112117
- DOI: 10.1128/JVI.79.11.7207-7216.2005
Poliovirus proteins induce membrane association of GTPase ADP-ribosylation factor
Abstract
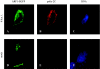
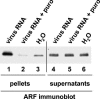
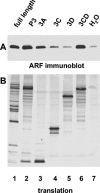
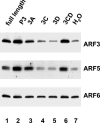
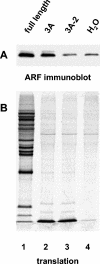
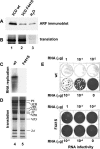
Poliovirus infection results in the disintegration of intracellular membrane structures and formation of specific vesicles that serve as sites for replication of viral RNA. The mechanism of membrane rearrangement has not been clearly defined. Replication of poliovirus is sensitive to brefeldin A (BFA), a fungal metabolite known to prevent normal function of the ADP-ribosylation factor (ARF) family of small GTPases. During normal membrane trafficking in uninfected cells, ARFs are involved in vesicle formation from different intracellular sites through interaction with numerous regulatory and coat proteins as well as in regulation of phospholipase D activity and cytoskeleton modifications. We demonstrate here that ARFs 3 and 5, but not ARF6, are translocated to membranes in HeLa cell extracts that are engaged in translation of poliovirus RNA. The accumulation of ARFs on membranes correlates with active replication of poliovirus RNA in vitro, whereas ARF translocation to membranes does not occur in the presence of BFA. ARF translocation can be induced independently by synthesis of poliovirus 3A or 3CD proteins, and we describe mutations that abolished this activity. In infected HeLa cells, an ARF1-enhanced green fluorescent protein fusion redistributes from Golgi stacks to the perinuclear region, where poliovirus RNA replication occurs. Taken together, the data suggest an involvement of ARF in poliovirus RNA replication.
Figures


References
-
- Aldabe, R., and L. Carrasco. 1995. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem. Biophys. Res. Commun. 206:64-76. - PubMed
-
- Balch, W. E., R. A. Kahn, and R. Schwaninger. 1992. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J. Biol. Chem. 267:13053-13061. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

