The integrins of the urochordate Ciona intestinalis provide novel insights into the molecular evolution of the vertebrate integrin family
- PMID: 15892888
- PMCID: PMC1145181
- DOI: 10.1186/1471-2148-5-31
The integrins of the urochordate Ciona intestinalis provide novel insights into the molecular evolution of the vertebrate integrin family
Abstract
Background: Integrins are a functionally significant family of metazoan cell surface adhesion receptors. The receptors are dimers composed of an alpha and a beta chain. Vertebrate genomes encode an expanded set of integrin alpha and beta chains in comparison with protostomes such as drosophila or the nematode worm. The publication of the genome of a basal chordate, Ciona intestinalis, provides a unique opportunity to gain further insight into how and when the expanded integrin supergene family found in vertebrates evolved.
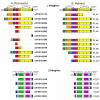
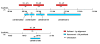
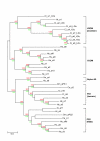
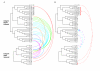
Results: The Ciona genome encodes eleven alpha and five beta chain genes that are highly homologous to their vertebrate homologues. Eight of the alpha chains contain an A-domain that lacks the short alpha helical region present in the collagen-binding vertebrate alpha chains. Phylogenetic analyses indicate the eight A-domain containing alpha chains cluster to form an ascidian-specific clade that is related to but, distinct from, the vertebrate A-domain clade. Two Ciona alpha chains cluster in laminin-binding clade and the remaining chain clusters in the clade that binds the RGD tripeptide sequence. Of the five Ciona beta chains, three form an ascidian-specific clade, one clusters in the vertebrate beta1 clade and the remaining Ciona chain is the orthologue of the vertebrate beta4 chain.
Conclusion: The Ciona repertoire of integrin genes provides new insight into the basic set of these receptors available at the beginning of vertebrate evolution. The ascidian and vertebrate alpha chain A-domain clades originated from a common precursor but radiated separately in each lineage. It would appear that the acquisition of collagen binding capabilities occurred in the chordate lineage after the divergence of ascidians.
Figures






References
-
- Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic-signalling pathways. J Cell Sci. 2001;114:2553–2560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

