Examination of the requirement for ucp-4, a putative homolog of mammalian uncoupling proteins, for stress tolerance and longevity in C. elegans
- PMID: 15893362
- PMCID: PMC2553218
- DOI: 10.1016/j.mad.2005.04.002
Examination of the requirement for ucp-4, a putative homolog of mammalian uncoupling proteins, for stress tolerance and longevity in C. elegans
Abstract
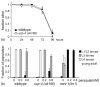
Reactive oxygen species (ROS) are generated by mitochondrial respiration and can react with and damage cellular components. According to the free radical theory of aging, oxidative damage from mitochondrial ROS is a major cause of cellular decline during aging. Mitochondrial uncoupling proteins (UCPs) uncouple ATP production from electron transport and can be stimulated by free radicals, suggesting UCPs may perform a cytoprotective function. The nematode, Caenorhabditis elegans, contains one UCP-like protein, encoded by the ucp-4 gene. We have investigated the genetic requirement for ucp-4 in normal aging and stress resistance. Consistent with the hypothesis that ucp-4 encodes a putative uncoupling protein, animals lacking ucp-4 function contained elevated ATP levels. However, the absence of ucp-4 function did not affect adult lifespan or survival in the presence of thermal or oxidative stress. Together, these results demonstrate that ucp-4 is a negative regulator of ATP production in C. elegans, but is not required for normal lifespan.
Figures


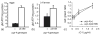

Similar articles
-
Uncoupling Protein, UCP-4 May Be Involved in Neuronal Defects During Aging and Resistance to Pathogens in Caenorhabditis elegans.Mol Cells. 2016 Sep;39(9):680-6. doi: 10.14348/molcells.2016.0125. Epub 2016 Sep 20. Mol Cells. 2016. PMID: 27646689 Free PMC article.
-
Uncoupling protein homologs may provide a link between mitochondria, metabolism and lifespan.Ageing Res Rev. 2006 May;5(2):196-208. doi: 10.1016/j.arr.2006.03.007. Epub 2006 May 16. Ageing Res Rev. 2006. PMID: 16707280 Free PMC article. Review.
-
Caenorhabditis elegans UCP4 protein controls complex II-mediated oxidative phosphorylation through succinate transport.J Biol Chem. 2011 Oct 28;286(43):37712-20. doi: 10.1074/jbc.M111.271452. Epub 2011 Aug 23. J Biol Chem. 2011. PMID: 21862587 Free PMC article.
-
Repression of the mitochondrial peroxiredoxin antioxidant system does not shorten life span but causes reduced fitness in Caenorhabditis elegans.Free Radic Biol Med. 2013 Oct;63:381-9. doi: 10.1016/j.freeradbiomed.2013.05.025. Epub 2013 May 28. Free Radic Biol Med. 2013. PMID: 23722165
-
Mitochondrial respiration and reactive oxygen species in C. elegans.Exp Gerontol. 2006 Oct;41(10):957-67. doi: 10.1016/j.exger.2006.06.056. Epub 2006 Aug 21. Exp Gerontol. 2006. PMID: 16919906 Review.
Cited by
-
Disruption of insulin signalling preserves bioenergetic competence of mitochondria in ageing Caenorhabditis elegans.BMC Biol. 2010 Jun 28;8:91. doi: 10.1186/1741-7007-8-91. BMC Biol. 2010. PMID: 20584279 Free PMC article.
-
Uncoupling Protein, UCP-4 May Be Involved in Neuronal Defects During Aging and Resistance to Pathogens in Caenorhabditis elegans.Mol Cells. 2016 Sep;39(9):680-6. doi: 10.14348/molcells.2016.0125. Epub 2016 Sep 20. Mol Cells. 2016. PMID: 27646689 Free PMC article.
-
Uncoupling protein homologs may provide a link between mitochondria, metabolism and lifespan.Ageing Res Rev. 2006 May;5(2):196-208. doi: 10.1016/j.arr.2006.03.007. Epub 2006 May 16. Ageing Res Rev. 2006. PMID: 16707280 Free PMC article. Review.
-
Expression of human uncoupling protein-3 in Drosophila insulin-producing cells increases insulin-like peptide (DILP) levels and shortens lifespan.Exp Gerontol. 2009 May;44(5):316-27. doi: 10.1016/j.exger.2009.02.001. Exp Gerontol. 2009. PMID: 19385039 Free PMC article.
-
UCP4C mediates uncoupled respiration in larvae of Drosophila melanogaster.EMBO Rep. 2014 May;15(5):586-91. doi: 10.1002/embr.201337972. Epub 2014 Mar 17. EMBO Rep. 2014. PMID: 24639557 Free PMC article.
References
-
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. - PubMed
-
- Braeckman B, Houthoofd K, De Vreese A, Vanfleteren J. Apparent uncoupling of energy production and consumption in long-lived Clk mutants of Caenorhabditis elegans. Curr Biol. 1999;9:493–496. - PubMed
-
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radical Biol Med. 2004;37:755–767. - PubMed
-
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. - PubMed
-
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

