Ontogeny of postsynaptic density proteins at glutamatergic synapses
- PMID: 15894489
- PMCID: PMC1414063
- DOI: 10.1016/j.mcn.2005.03.013
Ontogeny of postsynaptic density proteins at glutamatergic synapses
Abstract
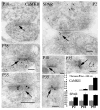
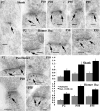
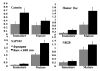
In glutamatergic synapses, glutamate receptors (GluRs) associate with many other proteins involved in scaffolding and signal transduction. The ontogeny of these postsynaptic density (PSD) proteins involves changes in their composition during development, paralleling changes in GluR type and function. In the CA1 region of the hippocampus, at postnatal day 2 (P2), many synapses already have a distinct PSD. We used immunoblot analysis, subcellular fractionation, and quantitative immunogold electron microscopy to examine the distribution of PSD proteins during development of the hippocampus. Synapses at P2 contained substantial levels of NR1 and NR2B and most GluR-associated proteins, including SAP102, SynGAP, the chain of proteins from GluRs/SAP102 through GKAP/Shank/Homer and metabotropic glutamate receptors, and the adhesion factors, cadherin, catenin, neuroligin, and Nr-CAM. Development was marked by substantial decreases in NR2B and SAP102 and increases in NR2A, PSD-95, AMPA receptors, and CaMKII. Other components showed more moderate changes.
Figures
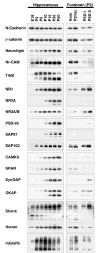

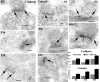
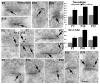

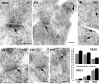

References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
-
- Alberts P, Galli T. The cell outgrowth secretory endosome (COSE): a specialized compartment involved in neuronal morphogenesis. Biol Cell. 2003;95:419–424. - PubMed
-
- Ango F, de Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at Purkinje axon initial segment. Cell. 2004;119:257–272. - PubMed
-
- Aoki C, Wu K, Elste A, Len GW, Lin SY, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. - PubMed
-
- Appel F, Holm J, Conscience J, von Bohlen und Halbach F, Faissner A, James P, Schachner M. Identification of the border between fibronectin type III homologous repeats 2 and 3 of the neural cell adhesion molecule L1 as a neurite outgrowth promoting and signal-transducing domain. J Neurobiol. 1995;28:297–312. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

