5-Lipoxygenase deficiency prevents respiratory failure during ventilator-induced lung injury
- PMID: 15894604
- PMCID: PMC2718472
- DOI: 10.1164/rccm.200501-034OC
5-Lipoxygenase deficiency prevents respiratory failure during ventilator-induced lung injury
Abstract
Rationale: Mechanical ventilation with high VT (HVT) progressively leads to lung injury and decreased efficiency of gas exchange. Hypoxic pulmonary vasoconstriction (HPV) directs blood flow to well-ventilated lung regions, preserving systemic oxygenation during pulmonary injury. Recent experimental studies have revealed an important role for leukotriene (LT) biosynthesis by 5-lipoxygenase (5LO) in the impairment of HPV by endotoxin.
Objectives: To investigate whether or not impairment of HPV contributes to the hypoxemia associated with HVT and to evaluate the role of LTs in ventilator-induced lung injury.
Methods: We studied wild-type and 5LO-deficient mice ventilated for up to 10 hours with low VT (LVT) or HVT.
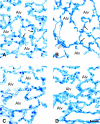
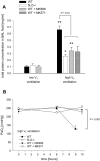
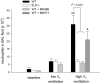
Results: In wild-type mice, HVT, but not LVT, increased pulmonary vascular permeability and edema formation, impaired systemic oxygenation, and reduced survival. HPV, as reflected by the increase in left pulmonary vascular resistance induced by left mainstem bronchus occlusion, was markedly impaired in animals ventilated with HVT. HVT ventilation increased bronchoalveolar lavage levels of LTs and neutrophils. In 5LO-deficient mice, the HVT-induced increase of pulmonary vascular permeability and worsening of respiratory mechanics were markedly attenuated, systemic oxygenation was preserved, and survival increased. Moreover, in 5LO-deficient mice, HVT ventilation did not impair the ability of left mainstem bronchus occlusion to increase left pulmonary vascular resistance. Administration of MK886, a 5LO-activity inhibitor, or MK571, a selective cysteinyl-LT(1) receptor antagonist, largely prevented ventilator-induced lung injury.
Conclusions: These results indicate that LTs play a central role in the lung injury and impaired oxygenation induced by HVT ventilation.
Figures





Comment in
-
Leukotrienes in acute lung injury: a potential therapeutic target?Am J Respir Crit Care Med. 2005 Aug 1;172(3):261-2. doi: 10.1164/rccm.2505008. Am J Respir Crit Care Med. 2005. PMID: 16040787 Free PMC article. No abstract available.
References
-
- Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures: protection by positive end-expiratory pressure. Am Rev Respir Dis 1974;110:556–565. - PubMed
-
- Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis 1985;132:880–884. - PubMed
-
- Kolobow T, Moretti MP, Fumagalli R, Mascheroni D, Prato P, Chen V, Joris M. Severe impairment in lung function induced by high peak airway pressure during mechanical ventilation: an experimental study. Am Rev Respir Dis 1987;135:312–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

