The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses
- PMID: 15894620
- PMCID: PMC1140425
- DOI: 10.1073/pnas.0500778102
The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses
Erratum in
- Proc Natl Acad Sci U S A. 2005 Jul 5;102(27):9734
Abstract
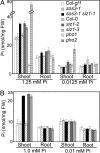
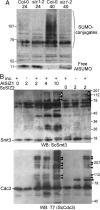
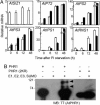
Plants sense phosphate (Pi) deficiency and initiate signaling that controls adaptive responses necessary for Pi acquisition. Herein, evidence establishes that AtSIZ1 is a plant small ubiquitin-like modifier (SUMO) E3 ligase and is a focal controller of Pi starvation-dependent responses. T-DNA insertional mutated alleles of AtSIZ1 (At5g60410) cause Arabidopsis to exhibit exaggerated prototypical Pi starvation responses, including cessation of primary root growth, extensive lateral root and root hair development, increase in root/shoot mass ratio, and greater anthocyanin accumulation, even though intracellular Pi levels in siz1 plants were similar to wild type. AtSIZ1 has SUMO E3 ligase activity in vitro, and immunoblot analysis revealed that the protein sumoylation profile is impaired in siz1 plants. AtSIZ1-GFP was localized to nuclear foci. Steadystate transcript abundances of Pi starvation-responsive genes AtPT2, AtPS2, and AtPS3 were moderate but clearly greater in siz1 seedlings than in wild type, where Pi is sufficient. Pi starvation induced the expression of these genes to the same extent in siz1 and wild-type seedlings. However, two other Pi starvation-responsive genes, AtIPS1 and AtRNS1, are induced more slowly in siz1 seedlings by Pi limitation. PHR1, a MYB transcriptional activator of AtIPS1 and AtRNS1, is an AtSIZ1 sumoylation target. These results indicate that AtSIZ1 is a SUMO E3 ligase and that sumoylation is a control mechanism that acts both negatively and positively on different Pi deficiency responses.
Figures


References
-
- Holford, I. C. R. (1997) Aust. J. Soil Res. 35, 227–239.
-
- Raghothama, K. G. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 665–693. - PubMed
-
- López-Bucio, J., Cruz-Ramírez, A. & Herrera-Estrella, L. (2003) Curr. Opin. Plant Biol. 6, 280–287. - PubMed
-
- Zakhleniuk, O. V., Raines, C. A. & Lloyd, J. C. (2001) Planta 212, 529–534. - PubMed
-
- Chen, D. L., Delatorre, C. A., Bakker, A. & Abel, S. (2000) Planta 211, 13–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

