Helix packing and orientation in the transmembrane dimer of gp55-P of the spleen focus forming virus
- PMID: 15894629
- PMCID: PMC1366604
- DOI: 10.1529/biophysj.104.057844
Helix packing and orientation in the transmembrane dimer of gp55-P of the spleen focus forming virus
Abstract
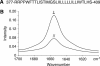
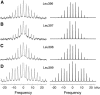
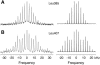
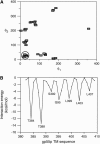
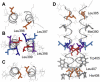
gp55-P is a dimeric membrane protein with a single transmembrane helix that is coded by the env gene of the polycythemic strain of the spleen focus forming virus. gp55-P activates the erythropoietin (Epo) receptor through specific transmembrane helix interactions, leading to Epo-independent growth of erythroid progenitors and eventually promoting erythroleukemia. We describe the use of magic angle spinning deuterium NMR to establish the structure of the transmembrane dimer of gp55-P in model membranes. Comparison of the deuterium lineshapes of leucines in the center (Leu(396-399)) and at the ends (Leu(385), Leu(407)) of the transmembrane sequence shows that gp55-P has a right-handed crossing angle with Leu(399) packed in the dimer interface. We discuss the implications of the structure of the gp55-P transmembrane dimer for activation of the Epo receptor.
Figures
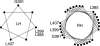
Similar articles
-
The erythropoietin receptor transmembrane domain mediates complex formation with viral anemic and polycythemic gp55 proteins.J Biol Chem. 2003 Oct 31;278(44):43755-63. doi: 10.1074/jbc.M302974200. Epub 2003 Aug 20. J Biol Chem. 2003. PMID: 12930840
-
Role of the transmembrane sequence of spleen focus-forming virus gp55 in erythroleukemogenesis.Virology. 1998 Dec 5;252(1):46-53. doi: 10.1006/viro.1998.9453. Virology. 1998. PMID: 9875316
-
Activation of the erythropoietin receptor by the gp55-P viral envelope protein is determined by a single amino acid in its transmembrane domain.EMBO J. 1999 Jun 15;18(12):3334-47. doi: 10.1093/emboj/18.12.3334. EMBO J. 1999. PMID: 10369674 Free PMC article.
-
Molecular mimicry of erythropoietin by the spleen focus-forming virus gp55 glycoprotein: the first stage of Friend virus-induced erythroleukemia.Biochim Biophys Acta. 1992 Sep 14;1114(1):31-41. doi: 10.1016/0304-419x(92)90004-i. Biochim Biophys Acta. 1992. PMID: 1390869 Review. No abstract available.
-
The interaction of the erythropoietin receptor and gp55.Cancer Surv. 1992;15:19-36. Cancer Surv. 1992. PMID: 1451111 Review.
Cited by
-
His499 Regulates Dimerization and Prevents Oncogenic Activation by Asparagine Mutations of the Human Thrombopoietin Receptor.J Biol Chem. 2016 Feb 5;291(6):2974-87. doi: 10.1074/jbc.M115.696534. Epub 2015 Dec 1. J Biol Chem. 2016. PMID: 26627830 Free PMC article.
-
An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor.Blood. 2006 Mar 1;107(5):1864-71. doi: 10.1182/blood-2005-06-2600. Epub 2005 Oct 25. Blood. 2006. PMID: 16249382 Free PMC article.
-
Coupling of transmembrane helix orientation to membrane release of the juxtamembrane region in FGFR3.Biochemistry. 2014 Aug 5;53(30):5000-7. doi: 10.1021/bi500327q. Epub 2014 Jul 24. Biochemistry. 2014. PMID: 25010350 Free PMC article.
-
Transmembrane helix orientation influences membrane binding of the intracellular juxtamembrane domain in Neu receptor peptides.Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1646-51. doi: 10.1073/pnas.1215207110. Epub 2013 Jan 14. Proc Natl Acad Sci U S A. 2013. PMID: 23319611 Free PMC article.
-
Tryptophan at the transmembrane-cytosolic junction modulates thrombopoietin receptor dimerization and activation.Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2540-5. doi: 10.1073/pnas.1211560110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359689 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

